Single Molecule Analysis of Laser Localized Interstrand Crosslinks
- PMID: 27242893
- PMCID: PMC4860505
- DOI: 10.3389/fgene.2016.00084
Single Molecule Analysis of Laser Localized Interstrand Crosslinks
Abstract
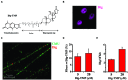
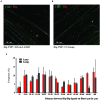
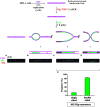
DNA interstrand crosslinks (ICLs) block unwinding of the double helix, and have always been regarded as major challenges to replication and transcription. Compounds that form these lesions are very toxic and are frequently used in cancer chemotherapy. We have developed two strategies, both based on immunofluorescence (IF), for studying cellular responses to ICLs. The basis of each is psoralen, a photoactive (by long wave ultraviolet light, UVA) DNA crosslinking agent, to which we have linked an antigen tag. In the one approach, we have taken advantage of DNA fiber and immuno-quantum dot technologies for visualizing the encounter of replication forks with ICLs induced by exposure to UVA lamps. In the other, psoralen ICLs are introduced into nuclei in live cells in regions of interest defined by a UVA laser. The antigen tag can be displayed by conventional IF, as can the recruitment and accumulation of DNA damage response proteins to the laser localized ICLs. However, substantial difference between the technologies creates considerable uncertainty as to whether conclusions from one approach are applicable to those of the other. In this report, we have employed the fiber/quantum dot methodology to determine lesion density and spacing on individual DNA molecules carrying laser localized ICLs. We have performed the same measurements on DNA fibers with ICLs induced by exposure of psoralen to UVA lamps. Remarkably, we find little difference in the adduct distribution on fibers prepared from cells exposed to the different treatment protocols. Furthermore, there is considerable similarity in patterns of replication in the vicinity of the ICLs introduced by the two techniques.
Keywords: DNA damage; DNA fiber; interstrand crosslinks; laser; replication; single molecule.
Figures


Similar articles
-
Single Molecule Analysis of Laser Localized Psoralen Adducts.J Vis Exp. 2017 Apr 20;(122):55541. doi: 10.3791/55541. J Vis Exp. 2017. PMID: 28448050 Free PMC article.
-
Quantification and repair of psoralen-induced interstrand crosslinks in human cells.Toxicol Lett. 2014 May 2;226(3):343-50. doi: 10.1016/j.toxlet.2014.01.019. Epub 2014 Feb 5. Toxicol Lett. 2014. PMID: 24508309
-
DNA interstrand crosslinks induce a potent replication block followed by formation and repair of double strand breaks in intact mammalian cells.DNA Repair (Amst). 2012 Dec 1;11(12):976-85. doi: 10.1016/j.dnarep.2012.09.010. Epub 2012 Oct 23. DNA Repair (Amst). 2012. PMID: 23099010
-
Using synthetic DNA interstrand crosslinks to elucidate repair pathways and identify new therapeutic targets for cancer chemotherapy.Cell Mol Life Sci. 2010 Nov;67(21):3683-97. doi: 10.1007/s00018-010-0492-6. Epub 2010 Aug 21. Cell Mol Life Sci. 2010. PMID: 20730555 Free PMC article. Review.
-
Cellular response to DNA interstrand crosslinks: the Fanconi anemia pathway.Cell Mol Life Sci. 2016 Aug;73(16):3097-114. doi: 10.1007/s00018-016-2218-x. Epub 2016 Apr 19. Cell Mol Life Sci. 2016. PMID: 27094386 Free PMC article. Review.
Cited by
-
The FANC/BRCA Pathway Releases Replication Blockades by Eliminating DNA Interstrand Cross-Links.Genes (Basel). 2020 May 25;11(5):585. doi: 10.3390/genes11050585. Genes (Basel). 2020. PMID: 32466131 Free PMC article. Review.
-
DONSON and FANCM associate with different replisomes distinguished by replication timing and chromatin domain.Nat Commun. 2020 Aug 7;11(1):3951. doi: 10.1038/s41467-020-17449-1. Nat Commun. 2020. PMID: 32769987 Free PMC article.
-
Single Molecule Analysis of Laser Localized Psoralen Adducts.J Vis Exp. 2017 Apr 20;(122):55541. doi: 10.3791/55541. J Vis Exp. 2017. PMID: 28448050 Free PMC article.
-
Imaging cellular responses to antigen tagged DNA damage.DNA Repair (Amst). 2018 Nov;71:183-189. doi: 10.1016/j.dnarep.2018.08.023. Epub 2018 Aug 23. DNA Repair (Amst). 2018. PMID: 30166246 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

