Cross Talk between Cancer and Mesenchymal Stem Cells through Extracellular Vesicles Carrying Nucleic Acids
- PMID: 27242964
- PMCID: PMC4876347
- DOI: 10.3389/fonc.2016.00125
Cross Talk between Cancer and Mesenchymal Stem Cells through Extracellular Vesicles Carrying Nucleic Acids
Abstract
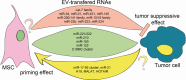
Extracellular vesicles (EVs) are considered to be a novel complex mechanism of cell communication within the tumor microenvironment. EVs may act as vehicles for transcription factors and nucleic acids inducing epigenetic changes in recipient cells. Since tumor EVs may be present in patient biological fluids, it is important to investigate their function and molecular mechanisms of action. It has been shown that tumor cells release EVs, which are capable of regulating cell apoptosis, proliferation, invasion, and epithelial-mesenchymal transition, as well as to suppress activity of immune cells, to enhance angiogenesis, and to prepare a favorable microenvironment for metastasis. On the other hand, EVs derived from stromal cells, such as mesenchymal stem cells (MSCs), may influence the phenotype of tumor cells through reciprocal cross talk greatly influenced by the transcription factors and nucleic acids they carry. In particular, non-coding RNAs (ncRNAs), including microRNAs and long ncRNAs, have recently been identified as the main candidates for the phenotypic changes induced in the recipient cells by EVs. ncRNAs, which are important regulators of mRNA and protein expression, can function either as tumor suppressors or as oncogenes, depending on their targets. Herein, we have attempted to revise actual evidence reported in the literature on the role of EVs in tumor biology with particular regard to the cross talk of ncRNAs between cancer cells and MSCs.
Keywords: exosomes; extracellular vesicles; long non-coding RNA; mesenchymal stem cells; microRNA; non-coding RNA; tumor stem cells.
Figures
References
-
- Lopatina T. The angiogenic potential of adipose mesenchymal stem cell-derived extracellular vesicles is modulated by basic fibroblast growth factor. J Stem Cell Res Ther (2014) 4(10):7. 10.4172/2157-7633.1000245 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

