Membrane Tethering Complexes in the Endosomal System
- PMID: 27243003
- PMCID: PMC4860415
- DOI: 10.3389/fcell.2016.00035
Membrane Tethering Complexes in the Endosomal System
Abstract
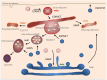
Vesicles that are generated by endocytic events at the plasma membrane are destined to early endosomes. A prerequisite for proper fusion is the tethering of two membrane entities. Tethering of vesicles to early endosomes is mediated by the class C core vacuole/endosome tethering (CORVET) complex, while fusion of late endosomes with lysosomes depends on the homotypic fusion and vacuole protein sorting (HOPS) complex. Recycling through the trans-Golgi network (TGN) and to the plasma membrane is facilitated by the Golgi associated retrograde protein (GARP) and endosome-associated recycling protein (EARP) complexes, respectively. However, there are other tethering functions in the endosomal system as there are multiple pathways through which proteins can be delivered from endosomes to either the TGN or the plasma membrane. Furthermore, proteins that may be part of novel tethering complexes have been recently identified. Thus, it is likely that more tethering factors exist. In this review, I will provide an overview of different tethering complexes of the endosomal system and discuss how they may provide specificity in membrane traffic.
Keywords: Golgi; endocytosis; exocytosis; membrane contact sites; membrane fusion; recycling; vesicle trafficking.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

