Differential effects of Cytomegalovirus carriage on the immune phenotype of middle-aged males and females
- PMID: 27243552
- PMCID: PMC4886678
- DOI: 10.1038/srep26892
Differential effects of Cytomegalovirus carriage on the immune phenotype of middle-aged males and females
Abstract
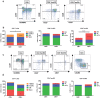
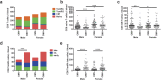
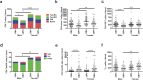
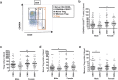
The elderly population is more susceptible to infections as a result of an altered immune response, commonly referred to as immunosenescence. Cytomegalovirus (CMV)-infection associated changes in blood lymphocytes are known to impact this process, but the interaction with gender remains unclear. Therefore, we analysed the effects and interaction of gender and CMV on the absolute numbers of a comprehensive set of naive and memory T- and B-cell subsets in people between 50 and 65 years of age. Enumeration and characterisation of lymphocyte subsets by flow cytometry was performed on fresh whole blood samples from 255 middle-aged persons. CMV-IgG serostatus was determined by ELISA. Gender was a major factor affecting immune cell numbers. CMV infection was mainly associated with an expansion of late-differentiated T-cell subsets. CMV+ males carried lower numbers of total CD4+, CD4+ central memory (CM) and follicular helper T-cells than females and CMV- males. Moreover, CMV+ males had significantly lower numbers of regulatory T (Treg)-cells and memory B-cells than CMV+ females. We here demonstrate an interaction between the effects of CMV infection and gender on T- and B-cells in middle-aged individuals. These differential effects on adaptive immunity between males and females may have implications for vaccination strategies at middle-age.
Conflict of interest statement
MCvZ reports grants from Erasmus MC, Rotterdam, the Netherlands, during the conduct of the study; In addition, MCvZ has a patent Detecting IgE-expressing B cells issued, and a patent Flow cytometric PID diagnostics pending. AMHB is a consultant for Grunenthal Gmbh (Germany) and was formerly employed (until October 2011) by MSD (Merck Research Laboratories in Oss, The Netherlands). All other authors have nothing to disclose.
Figures
References
-
- Weiskopf D., Weinberger B. & Grubeck-Loebenstein B. The aging of the immune system. Transplant international: official journal of the European Society for Organ Transplantation 22, 1041–1050 (2009). - PubMed
-
- Siegrist C. A. & Aspinall R. B-cell responses to vaccination at the extremes of age. Nature reviews. Immunology 9, 185–194 (2009). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

