Prices, Costs, and Affordability of New Medicines for Hepatitis C in 30 Countries: An Economic Analysis
- PMID: 27243629
- PMCID: PMC4886962
- DOI: 10.1371/journal.pmed.1002032
Prices, Costs, and Affordability of New Medicines for Hepatitis C in 30 Countries: An Economic Analysis
Abstract
Introduction: New hepatitis C virus (HCV) medicines have markedly improved treatment efficacy and regimen tolerability. However, their high prices have limited access, prompting wide debate about fair and affordable prices. This study systematically compared the price and affordability of sofosbuvir and ledipasvir/sofosbuvir across 30 countries to assess affordability to health systems and patients.
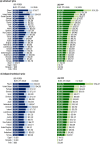
Methods and findings: Published 2015 ex-factory prices for a 12-wk course of treatment were provided by the Pharma Price Information (PPI) service of the Austrian public health institute Gesundheit Österreich GmbH or were obtained from national government or drug reimbursement authorities and recent press releases, where necessary. Prices in Organisation for Economic Co-operation and Development (OECD) member countries and select low- and middle-income countries were converted to US dollars using period average exchange rates and were adjusted for purchasing power parity (PPP). We analysed prices compared to national economic performance and estimated market size and the cost of these drugs in terms of countries' annual total pharmaceutical expenditure (TPE) and in terms of the duration of time an individual would need to work to pay for treatment out of pocket. Patient affordability was calculated using 2014 OECD average annual wages, supplemented with International Labour Organization median wage data where necessary. All data were compiled between 17 July 2015 and 25 January 2016. For the base case analysis, we assumed a 23% rebate/discount on the published price in all countries, except for countries with special pricing arrangements or generic licensing agreements. The median nominal ex-factory price of a 12-wk course of sofosbuvir across 26 OECD countries was US$42,017, ranging from US$37,729 in Japan to US$64,680 in the US. Central and Eastern European countries had higher PPP-adjusted prices than other countries: prices of sofosbuvir in Poland and Turkey (PPP$101,063 and PPP$70,331) and of ledipasvir/sofosbuvir in Poland (PPP$118,754) were at least 1.09 and 1.63 times higher, respectively than in the US (PPP$64,680 and PPP$72,765). Based on PPP-adjusted TPE and without the cost of ribavirin and other treatment costs, treating the entire HCV viraemic population with these regimens at the PPP-adjusted prices with a 23% price reduction would amount to at least one-tenth of current TPE across the countries included in this study, ranging from 10.5% of TPE in the Netherlands to 190.5% of TPE in Poland. In 12 countries, the price of a course of sofosbuvir without other costs was equivalent to 1 y or more of the average annual wage of individuals, ranging from 0.21 y in Egypt to 5.28 y in Turkey. This analysis relies on the accuracy of price information and infection prevalence estimates. It does not include the costs of diagnostic testing, supplementary treatments, treatment for patients with reinfection or cirrhosis, or associated health service costs.
Conclusions: Current prices of these medicines are variable and unaffordable globally. These prices threaten the sustainability of health systems in many countries and prevent large-scale provision of treatment. Stakeholders should implement a fairer pricing framework to deliver lower prices that take account of affordability. Without lower prices, countries are unlikely to be able to increase investment to minimise the burden of hepatitis C.
Conflict of interest statement
We have read the journal's policy and the authors of this manuscript have the following competing interests: SH is a member of the PLOS Medicine Editorial Board. KTT has been an employee of Deloitte Australia within the past 5 years. Deloitte Australia has provided consulting services to pharmaceutical companies. However, KTT has not been involved in any project directly related to the content of this manuscript that can lead to conflict of interest.
Figures



Comment in
-
A Revolution in Treatment for Hepatitis C Infection: Mitigating the Budgetary Impact.PLoS Med. 2016 May 31;13(5):e1002031. doi: 10.1371/journal.pmed.1002031. eCollection 2016 May. PLoS Med. 2016. PMID: 27243461 Free PMC article.
-
Hepatitis C drugs re-energize global fight over patents.Nature. 2017 Mar 1;543(7643):17-18. doi: 10.1038/543017a. Nature. 2017. PMID: 28252098 No abstract available.
References
-
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. 10.1016/S0140-6736(14)61682-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

