MitoNEET Protects HL-1 Cardiomyocytes from Oxidative Stress Mediated Apoptosis in an In Vitro Model of Hypoxia and Reoxygenation
- PMID: 27243905
- PMCID: PMC4887087
- DOI: 10.1371/journal.pone.0156054
MitoNEET Protects HL-1 Cardiomyocytes from Oxidative Stress Mediated Apoptosis in an In Vitro Model of Hypoxia and Reoxygenation
Abstract
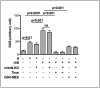
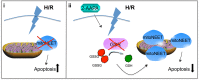
The iron-sulfur cluster containing protein mitoNEET is known to modulate the oxidative capacity of cardiac mitochondria but its function during myocardial reperfusion injury after transient ischemia is unknown. The purpose of this study was to analyze the impact of mitoNEET on oxidative stress induced cell death and its relation to the glutathione-redox system in cardiomyocytes in an in vitro model of hypoxia and reoxygenation (H/R). Our results show that siRNA knockdown (KD) of mitoNEET caused an 1.9-fold increase in H/R induced apoptosis compared to H/R control while overexpression of mitoNEET caused a 53% decrease in apoptosis. Necrosis was not affected. Apoptosis of both, mitoNEET-KD and control cells was diminished to comparable levels by using the antioxidants Tiron and glutathione compound glutathione reduced ethyl ester (GSH-MEE), indicating that mitoNEET-dependent apoptosis is mediated by oxidative stress. The interplay between mitoNEET and glutathione redox system was assessed by treating cardiomyocytes with 2-acetylamino-3-[4-(2-acetylamino-2-carboxyethylsulfanylthio-carbonylamino) phenylthiocarbamoylsulfanyl] propionic acid (2-AAPA), known to effectively inhibit glutathione reductase (GSR) and to decrease the GSH/GSSG ratio. Surprisingly, inhibition of GSR-activity to 20% by 2-AAPA decreased apoptosis of control and mitoNEET-KD cells to 23% and 25% respectively, while at the same time mitoNEET-protein was increased 4-fold. This effect on mitoNEET-protein was not accessible by mitoNEET-KD but was reversed by GSH-MEE. In conclusion we show that mitoNEET protects cardiomyocytes from oxidative stress-induced apoptosis during H/R. Inhibition of GSH-recycling, GSR-activity by 2-AAPA increased mitoNEET-protein, accompanied by reduced apoptosis. Addition of GSH reversed these effects suggesting that mitoNEET can in part compensate for imbalances in the antioxidative glutathione-system and therefore could serve as a potential therapeutic approach for the oxidatively stressed myocardium.
Conflict of interest statement
Figures





Similar articles
-
Evaluation of a dithiocarbamate derivative as a model of thiol oxidative stress in H9c2 rat cardiomyocytes.Free Radic Biol Med. 2014 May;70:214-22. doi: 10.1016/j.freeradbiomed.2014.02.022. Epub 2014 Mar 4. Free Radic Biol Med. 2014. PMID: 24607690 Free PMC article.
-
Impact of N-acetylcysteine on neonatal cardiomyocyte ischemia-reperfusion injury.Pediatr Res. 2011 Jul;70(1):61-6. doi: 10.1203/PDR.0b013e31821b1a92. Pediatr Res. 2011. PMID: 21427628
-
Effects of glutathione reductase inhibition on cellular thiol redox state and related systems.Arch Biochem Biophys. 2009 May 1;485(1):56-62. doi: 10.1016/j.abb.2009.03.001. Epub 2009 Mar 9. Arch Biochem Biophys. 2009. PMID: 19272349 Free PMC article.
-
Application of Glutathione as Anti-Oxidative and Anti-Aging Drugs.Curr Drug Metab. 2015;16(7):560-71. doi: 10.2174/1389200216666151015114515. Curr Drug Metab. 2015. PMID: 26467067 Review.
-
The Role of Non-Coding RNAs in the Neuroprotective Effects of Glutathione.Int J Mol Sci. 2021 Apr 19;22(8):4245. doi: 10.3390/ijms22084245. Int J Mol Sci. 2021. PMID: 33921907 Free PMC article. Review.
Cited by
-
MitoNEET Provides Cardioprotection via Reducing Oxidative Damage and Conserving Mitochondrial Function.Int J Mol Sci. 2023 Dec 29;25(1):480. doi: 10.3390/ijms25010480. Int J Mol Sci. 2023. PMID: 38203651 Free PMC article. Review.
-
Echo2Pheno: a deep-learning application to uncover echocardiographic phenotypes in conscious mice.Mamm Genome. 2023 Jun;34(2):200-215. doi: 10.1007/s00335-023-09996-x. Epub 2023 May 23. Mamm Genome. 2023. PMID: 37221250 Free PMC article.
-
Loss of the redox mitochondrial protein mitoNEET leads to mitochondrial dysfunction in B-cell acute lymphoblastic leukemia.Free Radic Biol Med. 2021 Nov 1;175:226-235. doi: 10.1016/j.freeradbiomed.2021.09.003. Epub 2021 Sep 5. Free Radic Biol Med. 2021. PMID: 34496224 Free PMC article.
-
Feasibility Analysis of Oxygen-Glucose Deprivation-Nutrition Resumption on H9c2 Cells In vitro Models of Myocardial Ischemia-Reperfusion Injury.Chin Med J (Engl). 2018 Oct 5;131(19):2277-2286. doi: 10.4103/0366-6999.241809. Chin Med J (Engl). 2018. PMID: 30246713 Free PMC article.
-
Collagen XIX Alpha 1 Improves Prognosis in Amyotrophic Lateral Sclerosis.Aging Dis. 2019 Apr 1;10(2):278-292. doi: 10.14336/AD.2018.0917. eCollection 2019 Apr. Aging Dis. 2019. PMID: 31011479 Free PMC article.
References
-
- Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, et al. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem. 1993;268(25):18532–41. Epub 1993/09/05. . - PubMed
-
- Park JW, Chun YS, Kim YH, Kim CH, Kim MS. Ischemic preconditioning reduces Op6 generation and prevents respiratory impairment in the mitochondria of post-ischemic reperfused heart of rat. Life Sci. 1997;60(24):2207–19. Epub 1997/01/01. S0024320597002361 [pii]. . - PubMed
-
- Tavazzi B, Di Pierro D, Bartolini M, Marino M, Distefano S, Galvano M, et al. Lipid peroxidation, tissue necrosis, and metabolic and mechanical recovery of isolated reperfused rat heart as a function of increasing ischemia. Free Radic Res. 1998;28(1):25–37. Epub 1998/04/29. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

