Olfactory Ensheathing Cell Transplantation in Experimental Spinal Cord Injury: Effect size and Reporting Bias of 62 Experimental Treatments: A Systematic Review and Meta-Analysis
- PMID: 27244556
- PMCID: PMC4886956
- DOI: 10.1371/journal.pbio.1002468
Olfactory Ensheathing Cell Transplantation in Experimental Spinal Cord Injury: Effect size and Reporting Bias of 62 Experimental Treatments: A Systematic Review and Meta-Analysis
Abstract
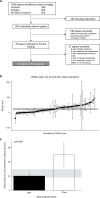
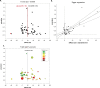
Olfactory ensheathing cell (OEC) transplantation is a candidate cellular treatment approach for human spinal cord injury (SCI) due to their unique regenerative potential and autologous origin. The objective of this study was, through a meta-epidemiologic approach, (i) to assess the efficacy of OEC transplantation on locomotor recovery after traumatic experimental SCI and (ii) to estimate the likelihood of reporting bias and/or missing data. A study protocol was finalized before data collection. Embedded into a systematic review and meta-analysis, we conducted a literature research of databases including PubMed, EMBASE, and ISI Web of Science from 1949/01 to 2014/10 with no language restrictions, screened by two independent investigators. Studies were included if they assessed neurobehavioral improvement after traumatic experimental SCI, administrated no combined interventions, and reported the number of animals in the treatment and control group. Individual effect sizes were pooled using a random effects model. Details regarding the study design were extracted and impact of these on locomotor outcome was assessed by meta-regression. Missing data (reporting bias) was determined by Egger regression and Funnel-plotting. The primary study outcome assessed was improvement in locomotor function at the final time point of measurement. We included 49 studies (62 experiments, 1,164 animals) in the final analysis. The overall improvement in locomotor function after OEC transplantation, measured using the Basso, Beattie, and Bresnahan (BBB) score, was 20.3% (95% CI 17.8-29.5). One missing study was imputed by trim and fill analysis, suggesting only slight publication bias and reducing the overall effect to a 19.2% improvement of locomotor activity. Dose-response ratio supports neurobiological plausibility. Studies were assessed using a 9-point item quality score, resulting in a median score of 5 (interquartile range [IQR] 3-5). In conclusion, OEC transplantation exerts considerable beneficial effects on neurobehavioral recovery after traumatic experimental SCI. Publication bias was minimal and affirms the translational potential of efficacy, but safety cannot be adequately assessed. The data justify OECs as a cellular substrate to develop and optimize minimally invasive and safe cellular transplantation paradigms for the lesioned spinal cord embedded into state-of-the-art Phase I/II clinical trial design studies for human SCI.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science (New York, NY). 1997;277(5334):2000–2. . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

