CD4 T Cell-Derived IFN-γ Plays a Minimal Role in Control of Pulmonary Mycobacterium tuberculosis Infection and Must Be Actively Repressed by PD-1 to Prevent Lethal Disease
- PMID: 27244558
- PMCID: PMC4887085
- DOI: 10.1371/journal.ppat.1005667
CD4 T Cell-Derived IFN-γ Plays a Minimal Role in Control of Pulmonary Mycobacterium tuberculosis Infection and Must Be Actively Repressed by PD-1 to Prevent Lethal Disease
Abstract
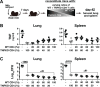
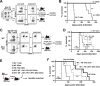
IFN-γ-producing CD4 T cells are required for protection against Mycobacterium tuberculosis (Mtb) infection, but the extent to which IFN-γ contributes to overall CD4 T cell-mediated protection remains unclear. Furthermore, it is not known if increasing IFN-γ production by CD4 T cells is desirable in Mtb infection. Here we show that IFN-γ accounts for only ~30% of CD4 T cell-dependent cumulative bacterial control in the lungs over the first six weeks of infection, but >80% of control in the spleen. Moreover, increasing the IFN-γ-producing capacity of CD4 T cells by ~2 fold exacerbates lung infection and leads to the early death of the host, despite enhancing control in the spleen. In addition, we show that the inhibitory receptor PD-1 facilitates host resistance to Mtb by preventing the detrimental over-production of IFN-γ by CD4 T cells. Specifically, PD-1 suppressed the parenchymal accumulation of and pathogenic IFN-γ production by the CXCR3+KLRG1-CX3CR1- subset of lung-homing CD4 T cells that otherwise mediates control of Mtb infection. Therefore, the primary role for T cell-derived IFN-γ in Mtb infection is at extra-pulmonary sites, and the host-protective subset of CD4 T cells requires negative regulation of IFN-γ production by PD-1 to prevent lethal immune-mediated pathology.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: DLB and AHS have patents related to PD-1. This does not alter our adherence to all PLOS Pathogens policies on sharing data and materials.
Figures




Similar articles
-
Protective CD4 T cells targeting cryptic epitopes of Mycobacterium tuberculosis resist infection-driven terminal differentiation.J Immunol. 2014 Apr 1;192(7):3247-58. doi: 10.4049/jimmunol.1300283. Epub 2014 Feb 26. J Immunol. 2014. PMID: 24574499
-
ESAT-6-dependent cytosolic pattern recognition drives noncognate tuberculosis control in vivo.J Clin Invest. 2016 Jun 1;126(6):2109-22. doi: 10.1172/JCI84978. Epub 2016 Apr 25. J Clin Invest. 2016. PMID: 27111234 Free PMC article.
-
IFN-γ from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection.J Immunol. 2013 Jan 1;190(1):270-7. doi: 10.4049/jimmunol.1200061. Epub 2012 Dec 10. J Immunol. 2013. PMID: 23233724 Free PMC article.
-
Innate and Adaptive Cellular Immune Responses to Mycobacterium tuberculosis Infection.Cold Spring Harb Perspect Med. 2015 Jul 17;5(12):a018424. doi: 10.1101/cshperspect.a018424. Cold Spring Harb Perspect Med. 2015. PMID: 26187873 Free PMC article. Review.
-
Defining features of protective CD4 T cell responses to Mycobacterium tuberculosis.Curr Opin Immunol. 2014 Aug;29:137-42. doi: 10.1016/j.coi.2014.06.003. Curr Opin Immunol. 2014. PMID: 25000593 Free PMC article. Review.
Cited by
-
Targeting innate immunity for tuberculosis vaccination.J Clin Invest. 2019 Sep 3;129(9):3482-3491. doi: 10.1172/JCI128877. J Clin Invest. 2019. PMID: 31478909 Free PMC article. Review.
-
Biomarkers and risk factors for the early prediction of immune-related adverse events: a review.Hum Vaccin Immunother. 2022 Dec 31;18(1):2018894. doi: 10.1080/21645515.2021.2018894. Epub 2022 Feb 2. Hum Vaccin Immunother. 2022. PMID: 35108160 Free PMC article. Review.
-
Host defense mechanisms against Mycobacterium tuberculosis.Cell Mol Life Sci. 2020 May;77(10):1859-1878. doi: 10.1007/s00018-019-03353-5. Epub 2019 Nov 13. Cell Mol Life Sci. 2020. PMID: 31720742 Free PMC article. Review.
-
NLRC3 negatively regulates CD4+ T cells and impacts protective immunity during Mycobacterium tuberculosis infection.PLoS Pathog. 2018 Aug 22;14(8):e1007266. doi: 10.1371/journal.ppat.1007266. eCollection 2018 Aug. PLoS Pathog. 2018. PMID: 30133544 Free PMC article.
-
Advances in Oncology in US and Japan: Focusing on Cancer and Infectious Diseases.World J Oncol. 2021 Dec;12(6):183-194. doi: 10.14740/wjon1422. Epub 2021 Dec 13. World J Oncol. 2021. PMID: 35059078 Free PMC article. Review.
References
-
- WHO. Global TB report 2015.
-
- Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AVS. Enhanced Immunogenicity and Protective Efficacy Against Mycobacterium tuberculosis of Bacille Calmette-Guerin Vaccine Using Mucosal Administration and Boosting with a Recombinant Modified Vaccinia Virus Ankara. The Journal of Immunology. 2003;171(3):1602–9. 10.4049/jimmunol.171.3.1602 - DOI - PubMed
-
- Wang J, Thorson L, Stokes RW, Santosuosso M, Huygen K, Zganiacz A, et al. Single Mucosal, but Not Parenteral, Immunization with Recombinant Adenoviral-Based Vaccine Provides Potent Protection from Pulmonary Tuberculosis. The Journal of Immunology. 2004;173(10):6357–65. 10.4049/jimmunol.173.10.6357 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

