Comprehensive Genomic Profiling Identifies a Subset of Crizotinib-Responsive ALK-Rearranged Non-Small Cell Lung Cancer Not Detected by Fluorescence In Situ Hybridization
- PMID: 27245569
- PMCID: PMC4912370
- DOI: 10.1634/theoncologist.2015-0497
Comprehensive Genomic Profiling Identifies a Subset of Crizotinib-Responsive ALK-Rearranged Non-Small Cell Lung Cancer Not Detected by Fluorescence In Situ Hybridization
Abstract
Introduction: For patients with non-small cell lung cancer (NSCLC) to benefit from ALK inhibitors, sensitive and specific detection of ALK genomic rearrangements is needed. ALK break-apart fluorescence in situ hybridization (FISH) is the U.S. Food and Drug Administration approved and standard-of-care diagnostic assay, but identification of ALK rearrangements by other methods reported in NSCLC cases that tested negative for ALK rearrangements by FISH suggests a significant false-negative rate. We report here a large series of NSCLC cases assayed by hybrid-capture-based comprehensive genomic profiling (CGP) in the course of clinical care.
Materials and methods: Hybrid-capture-based CGP using next-generation sequencing was performed in the course of clinical care of 1,070 patients with advanced lung cancer. Each tumor sample was evaluated for all classes of genomic alterations, including base-pair substitutions, insertions/deletions, copy number alterations and rearrangements, as well as fusions/rearrangements.
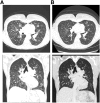
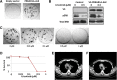
Results: A total of 47 patients (4.4%) were found to harbor ALK rearrangements, of whom 41 had an EML4-ALK fusion, and 6 had other fusion partners, including 3 previously unreported rearrangement events: EIF2AK-ALK, PPM1B-ALK, and PRKAR1A-ALK. Of 41 patients harboring ALK rearrangements, 31 had prior FISH testing results available. Of these, 20 were ALK FISH positive, and 11 (35%) were ALK FISH negative. Of the latter 11 patients, 9 received crizotinib based on the CGP results, and 7 achieved a response with median duration of 17 months.
Conclusion: Comprehensive genomic profiling detected canonical ALK rearrangements and ALK rearrangements with noncanonical fusion partners in a subset of patients with NSCLC with previously negative ALK FISH results. In this series, such patients had durable responses to ALK inhibitors, comparable to historical response rates for ALK FISH-positive cases.
Implications for practice: Comprehensive genomic profiling (CGP) that includes hybrid capture and specific baiting of intron 19 of ALK is a highly sensitive, alternative method for identification of drug-sensitive ALK fusions in patients with non-small cell lung cancer (NSCLC) who had previously tested negative using standard ALK fluorescence in situ hybridization (FISH) diagnostic assays. Given the proven benefit of treatment with crizotinib and second-generation ALK inhibitors in patients with ALK fusions, CGP should be considered in patients with NSCLC, including those who have tested negative for other alterations, including negative results using ALK FISH testing.
摘要
引言. 敏感且特异性地检测到ALK基因组重排为能够从ALK抑制剂治疗获益的非小细胞肺癌 (NSCLC) 患者所必需。ALK分离荧光原位杂交 (FISH) 是获得美国食品和药物管理局批准的标准诊断方法, 但使用其他方法在ALK重排FISH阴性的NSCLC病例中发现了ALK重排, 提示这种方法的假阴性率相当高。本文报告了在临床治疗过程中采用基于杂交捕获法的综合基因组分析 (CGP) 在一个NSCLC病例大型队列中进行检测的结果。
材料与方法. 临床诊疗过程中, 在1 070例晚期肺癌患者中使用应用下一代测序技术的基于杂交捕获法的CGP。对每个肿瘤标本都进行所有种类基因改变的评价, 包括碱基替换、插入/缺失、拷贝数改变和重排, 以及融合/重排。
结果. 共发现47例患者 (4.4%) 携带ALK重排, 其中41例有EML4-ALK融合, 其余6例为其他融合配偶体, 包括3例过去未报告的重排事件: EIF2AK-ALK、PPM1B-ALK和PRKAR1A-ALK。在41例携带ALK重排的患者中, 31例有既往FISH检验结果。其中20例为ALK FISH阳性, 11例 (35%) 为ALK FISH阴性。在后面这11例患者中, 9例依据CGP结果接受了克唑替尼治疗, 其中7例中位缓解时间达到17个月。
结论. 综合基因组分析在既往ALK FISH检验结果为阴性的NSCLC亚组患者中发现了经典ALK重排和非经典融合配偶体。在这一病例系列中, 此类患者对ALK抑制剂治疗产生了持久应答, 缓解率与ALK FISH阳性病例的历史缓解率相当。The Oncologist 2016;21:762–770
对临床实践的提示: 综合基因组分析 (CGP) 包括杂交捕获法以及 ALK 内含子 19 的特异性诱饵, 是在使用标准 ALK 荧光原位杂交法 (FISH) 诊断为阴性的非小细胞肺癌 (NSCLC) 患者中, 鉴别药物敏感性 ALK 融合的可选方法。鉴于已证实克唑替尼和第二代 ALK 抑制剂治疗在 ALK 融合患者中的获益, 应考虑对 NSCLC 患者使用 CGP 检测, 包括那些其他可选方法检验结果阴性的患者 (也包括使用 ALK FISH 检验结果阴性的患者)。
Keywords: ALK; Crizotinib; Fluorescence in situ hybridization; Fusion; Genomic profiling.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures




Comment in
-
Screening for ALK Rearrangements in Lung Cancer: Time for a New Generation of Diagnostics?Oncologist. 2016 Jun;21(6):662-3. doi: 10.1634/theoncologist.2016-0179. Epub 2016 May 31. Oncologist. 2016. PMID: 27245570 Free PMC article.
References
-
- US Food and Drug Administration. List of cleared or approved companion diagnostic devices (in vitro and imaging tools). Available at http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDi.... Accessed August 22, 2015.
-
- Heuckmann JM, Balke-Want H, Malchers F, et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res. 2012;18:4682–4690. - PubMed
-
- Shaw AT, Kim D-W, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

