Endogenous analgesia mediated by CD4(+) T lymphocytes is dependent on enkephalins in mice
- PMID: 27245576
- PMCID: PMC4888630
- DOI: 10.1186/s12974-016-0591-x
Endogenous analgesia mediated by CD4(+) T lymphocytes is dependent on enkephalins in mice
Abstract
Background: T cell-derived opioids play a key role in the control of inflammatory pain. However, the nature of opioids produced by T cells is still matter of debate in mice. Whereas β-endorphin has been found in T lymphocytes by using antibody-based methods, messenger RNA (mRNA) quantification shows mainly mRNA encoding for enkephalins. The objective of the study is to elucidate the nature of T cell-derived opioids responsible for analgesia and clarify discrepancy of the results at the protein and genetic levels.
Methods: CD4(+) T lymphocytes were isolated from wild-type and enkephalin-deficient mice. mRNA encoding for β-endorphin and enkephalin was quantified by RT-qPCR. The binding of commercially available polyclonal anti-endorphin antibodies to lymphocytes from wild-type or enkephalin knockout mice was assessed by cytofluorometry. Opioid-mediated analgesic properties of T lymphocytes from wild-type and enkephalin-deficient mice were compared in a model of inflammation-induced somatic pain by measuring sensitivity to mechanical stimuli using calibrated von Frey filaments.
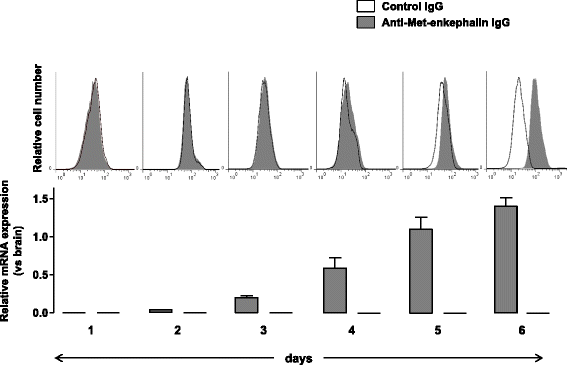
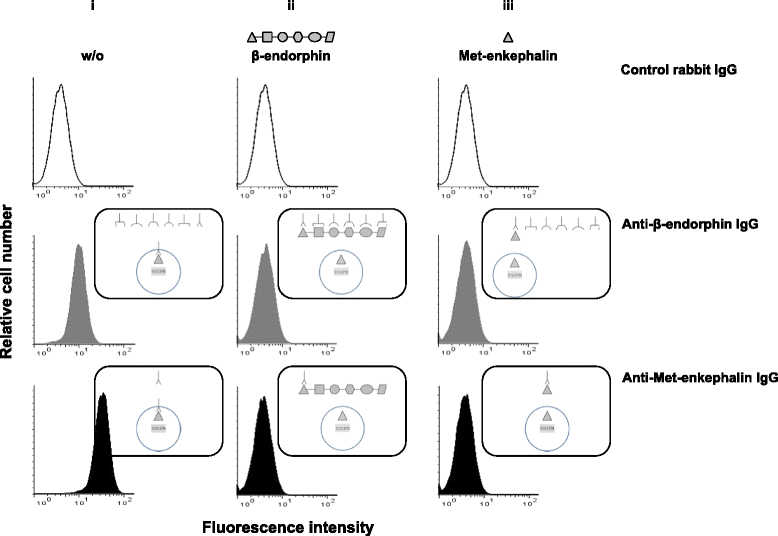
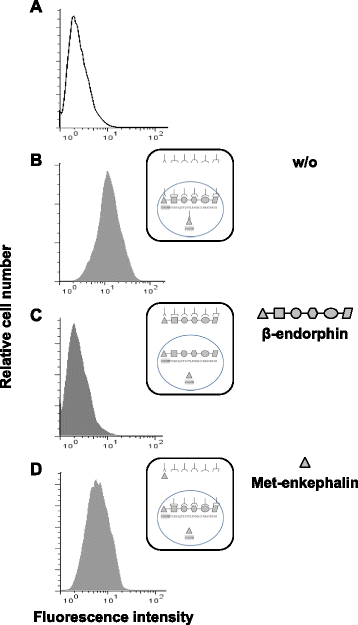
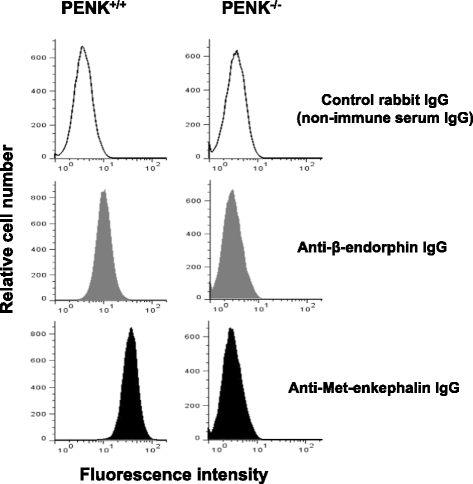
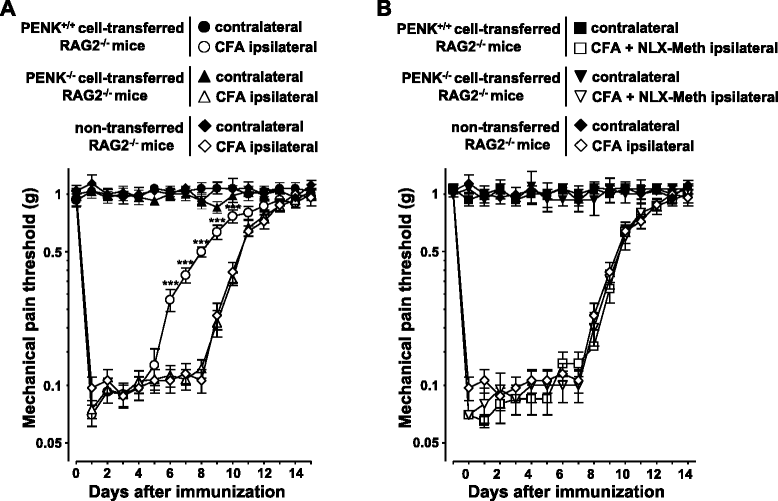
Results: CD4(+) T lymphocytes expressed high level of mRNA encoding for enkephalins but not for β-endorphin in mice. Anti-β-endorphin polyclonal IgG antibodies are specific for β-endorphin but cross-react with enkephalins. Anti-β-endorphin polyclonal antibodies bound to wild-type but not enkephalin-deficient CD4(+) T lymphocytes. Endogenous regulation of inflammatory pain by wild-type T lymphocytes was completely abolished when T lymphocytes were deficient in enkephalins. Pain behavior of immune-deficient (i.e., without B and T lymphocytes) mice was superimposable to that of mice transferred with enkephalin-deficient lymphocytes.
Conclusions: Rabbit polyclonal anti-β-endorphin serum IgG bind to CD4(+) T lymphocytes because of their cross-reactivity towards enkephalins. Thus, staining of T lymphocytes by anti-β-endorphin polyclonal IgG reported in most of studies in mice is because of their binding to enkephalins. In mice, CD4(+) T lymphocytes completely lose their analgesic opioid-mediated activity when lacking enkephalins.
Keywords: Enkephalin; Inflammation; Pain; T lymphocytes; β-endorphin.
Figures

 ) or β-endorphin (
) or β-endorphin ( )
)
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

