Germline or somatic GPR101 duplication leads to X-linked acrogigantism: a clinico-pathological and genetic study
- PMID: 27245663
- PMCID: PMC4888203
- DOI: 10.1186/s40478-016-0328-1
Germline or somatic GPR101 duplication leads to X-linked acrogigantism: a clinico-pathological and genetic study
Abstract
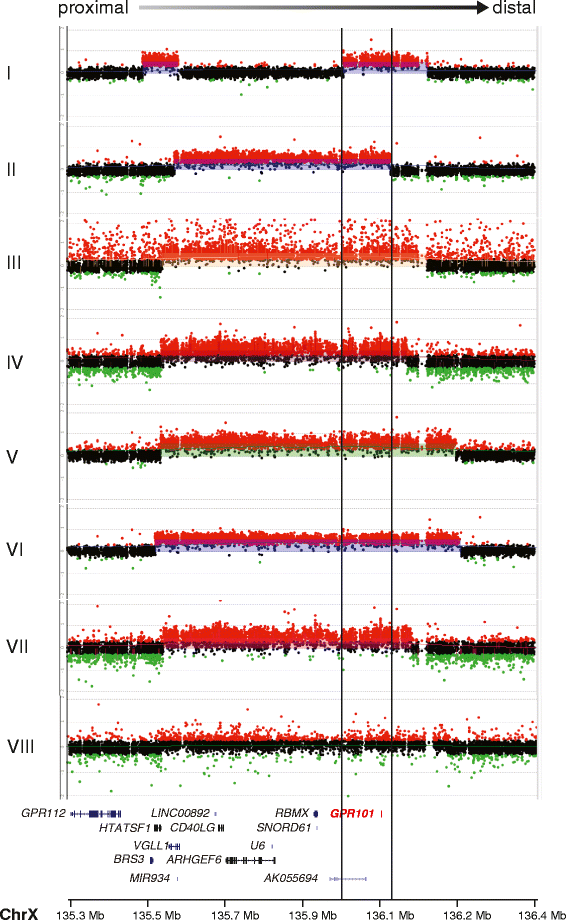
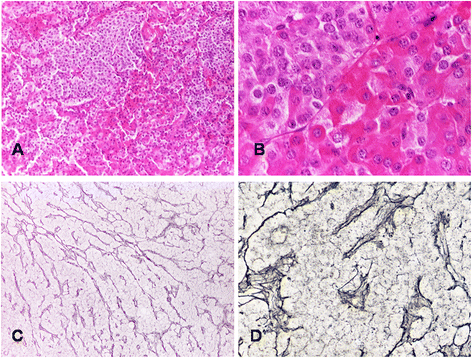
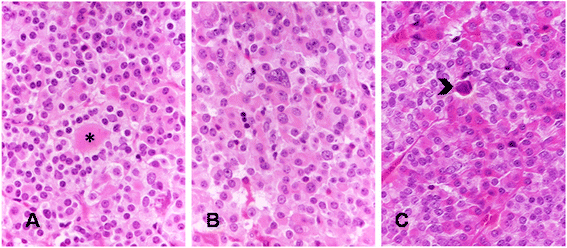
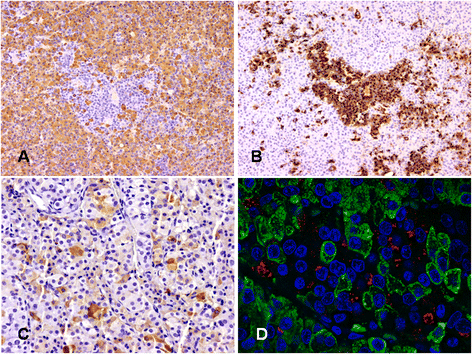
Non-syndromic pituitary gigantism can result from AIP mutations or the recently identified Xq26.3 microduplication causing X-linked acrogigantism (XLAG). Within Xq26.3, GPR101 is believed to be the causative gene, and the c.924G > C (p.E308D) variant in this orphan G protein-coupled receptor has been suggested to play a role in the pathogenesis of acromegaly.We studied 153 patients (58 females and 95 males) with pituitary gigantism. AIP mutation-negative cases were screened for GPR101 duplication through copy number variation droplet digital PCR and high-density aCGH. The genetic, clinical and histopathological features of XLAG patients were studied in detail. 395 peripheral blood and 193 pituitary tumor DNA samples from acromegaly patients were tested for GPR101 variants.We identified 12 patients (10 females and 2 males; 7.8 %) with XLAG. In one subject, the duplicated region only contained GPR101, but not the other three genes in found to be duplicated in the previously reported patients, defining a new smallest region of overlap of duplications. While females presented with germline mutations, the two male patients harbored the mutation in a mosaic state. Nine patients had pituitary adenomas, while three had hyperplasia. The comparison of the features of XLAG, AIP-positive and GPR101&AIP-negative patients revealed significant differences in sex distribution, age at onset, height, prolactin co-secretion and histological features. The pathological features of XLAG-related adenomas were remarkably similar. These tumors had a sinusoidal and lobular architecture. Sparsely and densely granulated somatotrophs were admixed with lactotrophs; follicle-like structures and calcifications were commonly observed. Patients with sporadic of familial acromegaly did not have an increased prevalence of the c.924G > C (p.E308D) GPR101 variant compared to public databases.In conclusion, XLAG can result from germline or somatic duplication of GPR101. Duplication of GPR101 alone is sufficient for the development of XLAG, implicating it as the causative gene within the Xq26.3 region. The pathological features of XLAG-associated pituitary adenomas are typical and, together with the clinical phenotype, should prompt genetic testing.
Trial registration: ClinicalTrials.gov NCT00461188.
Keywords: CNV mutation; GPR101; Gigantism; Pituitary; XLAG.
Figures
References
-
- Trivellin G, Daly AF, Faucz FR, Yuan B, Rostomyan L, Larco DO, Schernthaner-Reiter MH, Szarek E, Leal LF, Caberg JH, Castermans E, Villa C, Dimopoulos A, Chittiboina P, Xekouki P, Shah N, Metzger D, Lysy PA, Ferrante E, Strebkova N, Mazerkina N, Zatelli MC, Lodish M, Horvath A, de Alexandre RB, Manning AD, Levy I, Keil MF, Sierra Mde L, Palmeira L, Coppieters W, Georges M, Naves LA, Jamar M, Bours V, Wu TJ, Choong CS, Bertherat J, Chanson P, Kamenicky P, Farrell WE, Barlier A, Quezado M, Bjelobaba I, Stojilkovic SS, Wess J, Costanzi S, Liu P, Lupski JR, Beckers A, Stratakis CA. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med. 2014;371:2363–74. doi: 10.1056/NEJMoa1408028. - DOI - PMC - PubMed
-
- Beckers A, Lodish MB, Trivellin G, Rostomyan L, Lee M, Faucz FR, Yuan B, Choong CS, Caberg JH, Verrua E, Naves LA, Cheetham TD, Young J, Lysy PA, Petrossians P, Cotterill A, Shah NS, Metzger D, Castermans E, Ambrosio MR, Villa C, Strebkova N, Mazerkina N, Gaillard S, Barra GB, Casulari LA, Neggers SJ, Salvatori R, Jaffrain-Rea ML, Zacharin M, Santamaria BL, Zacharieva S, Lim EM, Mantovani G, Zatelli MC, Collins MT, Bonneville JF, Quezado M, Chittiboina P, Oldfield EH, Bours V, Liu P, WdH W, Pellegata N, Lupski JR, Daly AF, Stratakis CA. X-linked acrogigantism syndrome: clinical profile and therapeutic responses. Endocr Relat Cancer. 2015;22:353–67. doi: 10.1530/ERC-15-0038. - DOI - PMC - PubMed
-
- Hérnandez-Ramírez LC, Gabrovska P, Denes J, Stals K, Trivellin G, Tilley D, Ferraù F, Evanson J, Ellard S, Grossman AB, Roncaroli F, Gadelha MR, Korbonits M, International FC. Landscape of familial isolated and young-onset pituitary adenomas: prospective diagnosis in AIP mutation carriers. J Clin Endocrinol Metab. 2015;100:E1242–54. doi: 10.1210/jc.2015-1869. - DOI - PMC - PubMed
-
- Cuny T, Pertuit M, Sahnoun-Fathallah M, Daly A, Occhi G, Odou MF, Tabarin A, Nunes ML, Delemer B, Rohmer V, Desailloud R, Kerlan V, Chabre O, Sadoul JL, Cogne M, Caron P, Cortet-Rudelli C, Lienhardt A, Raingeard I, Guedj AM, Brue T, Beckers A, Weryha G, Enjalbert A, Barlier A. Genetic analysis in young patients with sporadic pituitary macroadenomas: besides AIP don't forget MEN1 genetic analysis. Eur J Endocrinol. 2013;168:533–41. doi: 10.1530/EJE-12-0763. - DOI - PubMed
-
- Rostomyan L, Daly AF, Petrossians P, Nachev E, Lila AR, Lecoq AL, Lecumberri B, Trivellin G, Salvatori R, Moraitis AG, Holdaway I, Kranenburg-van Klaveren DJ, Chiara Zatelli M, Palacios N, Nozieres C, Zacharin M, Ebeling T, Ojaniemi M, Rozhinskaya L, Verrua E, Jaffrain-Rea ML, Filipponi S, Gusakova D, Pronin V, Bertherat J, Belaya Z, Ilovayskaya I, Sahnoun-Fathallah M, Sievers C, Stalla GK, Castermans E, Caberg JH, Sorkina E, Auriemma RS, Mittal S, Kareva M, Lysy PA, Emy P, De Menis E, Choong CS, Mantovani G, Bours V, De Herder W, Brue T, Barlier A, Neggers SJ, Zacharieva S, Chanson P, Shah NS, Stratakis CA, Naves LA, Beckers A. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr Relat Cancer. 2015;22:745–57. doi: 10.1530/ERC-15-0320. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

