Hypoxia and tissue destruction in pulmonary TB
- PMID: 27245780
- PMCID: PMC5136721
- DOI: 10.1136/thoraxjnl-2015-207402
Hypoxia and tissue destruction in pulmonary TB
Abstract
Background: It is unknown whether lesions in human TB are hypoxic or whether this influences disease pathology. Human TB is characterised by extensive lung destruction driven by host matrix metalloproteinases (MMPs), particularly collagenases such as matrix metalloproteinase-1 (MMP-1).
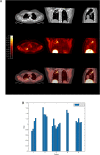
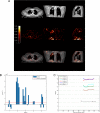
Methods: We investigated tissue hypoxia in five patients with PET imaging using the tracer [18F]-fluoromisonidazole ([18F]FMISO) and by immunohistochemistry. We studied the regulation of MMP secretion in primary human cell culture model systems in normoxia, hypoxia, chemical hypoxia and by small interfering RNA (siRNA) inhibition.
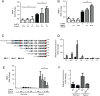
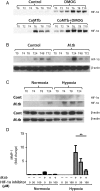
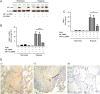
Results: [18F]FMISO accumulated in regions of TB consolidation and around pulmonary cavities, demonstrating for the first time severe tissue hypoxia in man. Patlak analysis of dynamic PET data showed heterogeneous levels of hypoxia within and between patients. In Mycobacterium tuberculosis (M.tb)-infected human macrophages, hypoxia (1% pO2) upregulated MMP-1 gene expression 170-fold, driving secretion and caseinolytic activity. Dimethyloxalyl glycine (DMOG), a small molecule inhibitor which stabilises the transcription factor hypoxia-inducible factor (HIF)-1α, similarly upregulated MMP-1. Hypoxia did not affect mycobacterial replication. Hypoxia increased MMP-1 expression in primary respiratory epithelial cells via intercellular networks regulated by TB. HIF-1α and NF-κB regulated increased MMP-1 activity in hypoxia. Furthermore, M.tb infection drove HIF-1α accumulation even in normoxia. In human TB lung biopsies, epithelioid macrophages and multinucleate giant cells express HIF-1α. HIF-1α blockade, including by targeted siRNA, inhibited TB-driven MMP-1 gene expression and secretion.
Conclusions: Human TB lesions are severely hypoxic and M.tb drives HIF-1α accumulation, synergistically increasing collagenase activity which will lead to lung destruction and cavitation.
Keywords: Tuberculosis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- WHO. Global Tuberculosis Report 2012. 2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
