STIMs and Orai1 regulate cytokine production in spinal astrocytes
- PMID: 27245842
- PMCID: PMC4886427
- DOI: 10.1186/s12974-016-0594-7
STIMs and Orai1 regulate cytokine production in spinal astrocytes
Abstract
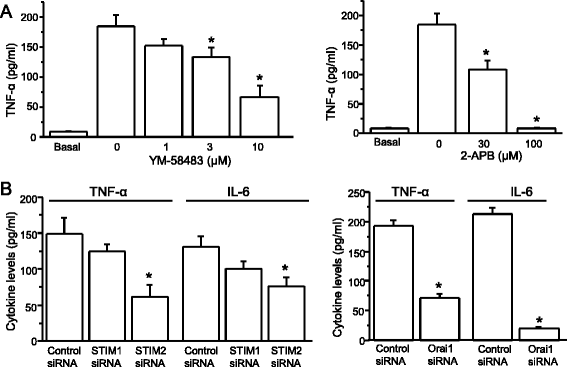
Background: Our previous study demonstrated that a store-operated calcium channel (SOCC) inhibitor (YM-58483) has central analgesic effects. However, the cellular and molecular mechanisms of such effects remain to be determined. It is well-known that glial cells play important roles in central sensitization. SOC entry (SOCE) has been implicated in many cell types including cortical astrocytes. However, the role of the SOCC family in the function of astrocytes has not been determined. Here, we thoroughly investigated the expression and the functional significance of SOCCs in spinal astrocytes.
Methods: Primary cultured astrocytes were prepared from neonatal (P2-P3) CD1 mice. Expressions of mRNAs and proteins were respectively assessed by real-time PCR and Western blot analysis. SOCE was measured using a calcium imaging system. Live-cell STIM1 translocation was detected using a confocal microscope. Cytokine levels were measured by the enzyme-linked immunosorbent assay.
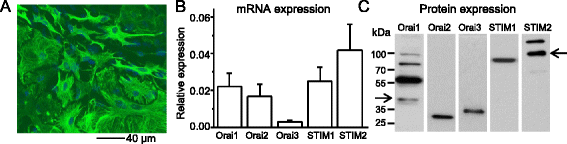
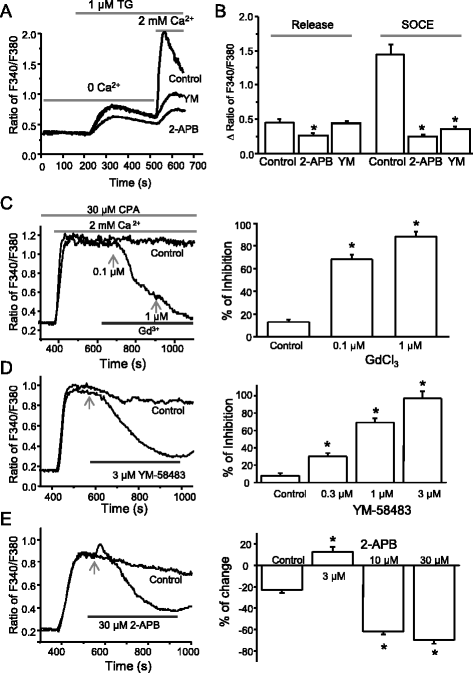
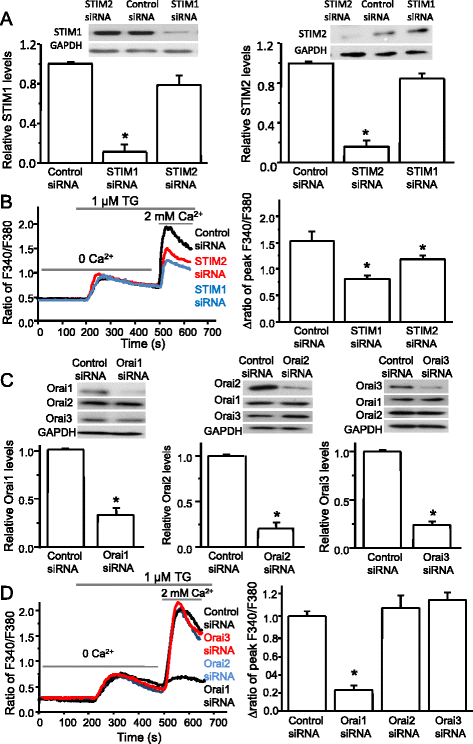
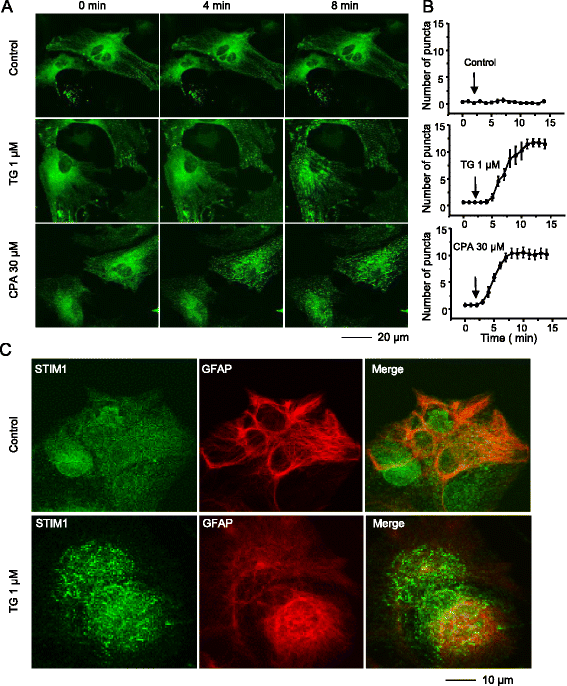
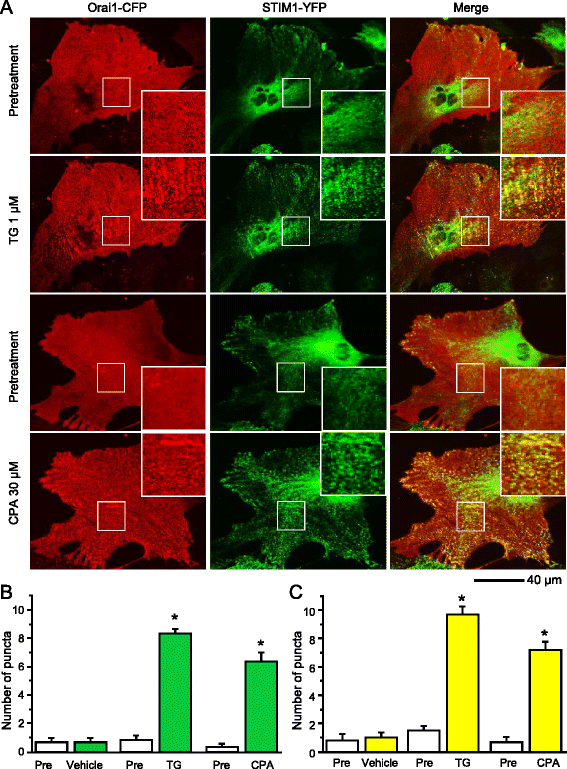
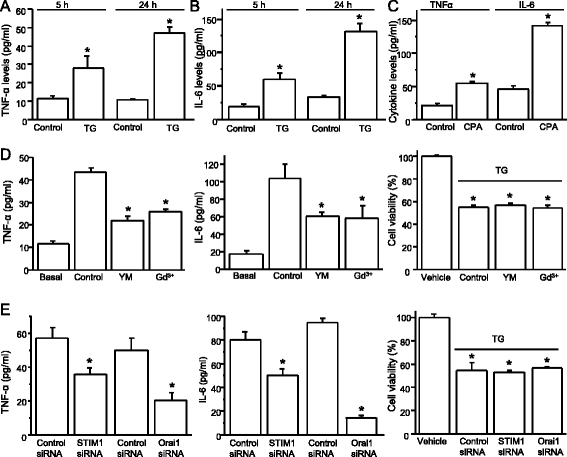
Results: We found that the SOCC family is expressed in spinal astrocytes and that depletion of calcium stores from the endoplasmic reticulum by cyclopiazonic acid (CPA) resulted in a large sustained calcium entry, which was blocked by SOCC inhibitors. Using the siRNA knockdown approach, we identified STIM1 and Orai1 as primary components of SOCCs in spinal astrocytes. We also observed thapsigargin (TG)- or CPA-induced puncta formation of STIM1 and Orai1. In addition, activation of SOCCs remarkably promoted TNF-α and IL-6 production in spinal astrocytes, which were greatly attenuated by knockdown of STIM1 or Orai1. Importantly, knockdown of STIM2 and Orai1 dramatically decreased lipopolysaccharide-induced TNF-α and IL-6 production without changing cell viability.
Conclusions: This study presents the first evidence that STIM1, STIM2, and Orai1 mediate SOCE and are involved in cytokine production in spinal astrocytes. Our findings provide the basis for future assessment of SOCCs in pain and other central nervous system disorders associated with abnormal astrocyte activities.
Keywords: Astrocytes; Cytokine; Orai1; STIM1; Store-operated calcium channels; The spinal cord.
Figures
Similar articles
-
Regulation of phagocytosis and cytokine secretion by store-operated calcium entry in primary isolated murine microglia.Cell Signal. 2015 Jan;27(1):177-86. doi: 10.1016/j.cellsig.2014.11.003. Epub 2014 Nov 7. Cell Signal. 2015. PMID: 25451082
-
STIM1 and Orai1 mediate thrombin-induced Ca(2+) influx in rat cortical astrocytes.Cell Calcium. 2012 Dec;52(6):457-67. doi: 10.1016/j.ceca.2012.08.004. Epub 2012 Sep 1. Cell Calcium. 2012. PMID: 22944608
-
Native store-operated calcium channels are functionally expressed in mouse spinal cord dorsal horn neurons and regulate resting calcium homeostasis.J Physiol. 2014 Aug 15;592(16):3443-61. doi: 10.1113/jphysiol.2014.275065. Epub 2014 May 23. J Physiol. 2014. PMID: 24860175 Free PMC article.
-
STIM-TRP Pathways and Microdomain Organization: Contribution of TRPC1 in Store-Operated Ca2+ Entry: Impact on Ca2+ Signaling and Cell Function.Adv Exp Med Biol. 2017;993:159-188. doi: 10.1007/978-3-319-57732-6_9. Adv Exp Med Biol. 2017. PMID: 28900914 Review.
-
Store-operated Ca(2+) entry.Adv Exp Med Biol. 2012;740:349-82. doi: 10.1007/978-94-007-2888-2_15. Adv Exp Med Biol. 2012. PMID: 22453950 Review.
Cited by
-
Cell-wide mapping of Orai1 channel activity reveals functional heterogeneity in STIM1-Orai1 puncta.J Gen Physiol. 2020 Sep 7;152(9):e201812239. doi: 10.1085/jgp.201812239. J Gen Physiol. 2020. PMID: 32589186 Free PMC article.
-
Toll-like receptor 4 activation enhances Orai1-mediated calcium signal promoting cytokine production in spinal astrocytes.Cell Calcium. 2022 Jul;105:102619. doi: 10.1016/j.ceca.2022.102619. Epub 2022 Jun 25. Cell Calcium. 2022. PMID: 35780680 Free PMC article.
-
Insights Into Spinal Dorsal Horn Circuit Function and Dysfunction Using Optical Approaches.Front Neural Circuits. 2020 Jun 12;14:31. doi: 10.3389/fncir.2020.00031. eCollection 2020. Front Neural Circuits. 2020. PMID: 32595458 Free PMC article. Review.
-
Role of STIM2 in cell function and physiopathology.J Physiol. 2017 May 15;595(10):3111-3128. doi: 10.1113/JP273889. Epub 2017 Feb 19. J Physiol. 2017. PMID: 28087881 Free PMC article. Review.
-
Ca2+-dependent endoplasmic reticulum stress correlation with astrogliosis involves upregulation of KCa3.1 and inhibition of AKT/mTOR signaling.J Neuroinflammation. 2018 Nov 15;15(1):316. doi: 10.1186/s12974-018-1351-x. J Neuroinflammation. 2018. PMID: 30442153 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

