Multimodal interaction in the insect brain
- PMID: 27246183
- PMCID: PMC4888552
- DOI: 10.1186/s12868-016-0258-7
Multimodal interaction in the insect brain
Abstract
Background: The magnitude of multimodal enhancement in the brain is believed to depend on the stimulus intensity and timing. Such an effect has been found in many species, but has not been previously investigated in insects.
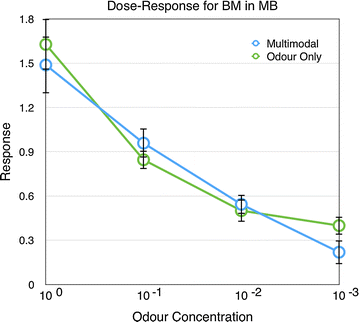
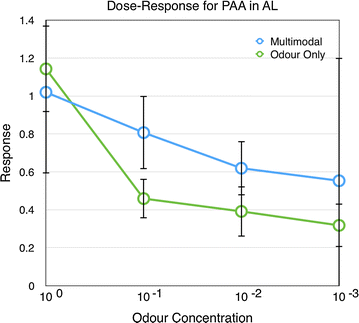
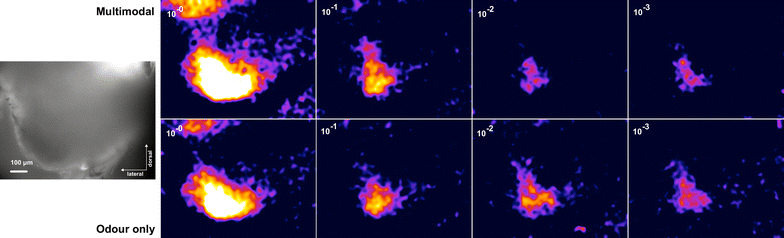
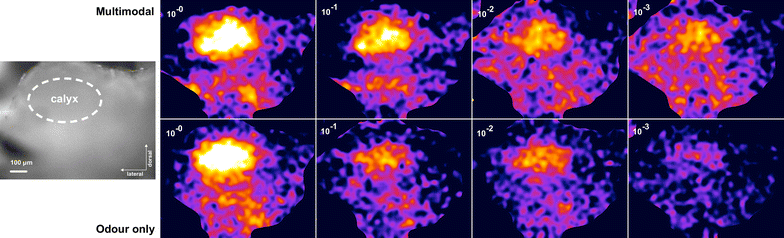
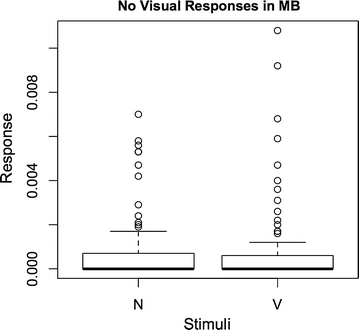
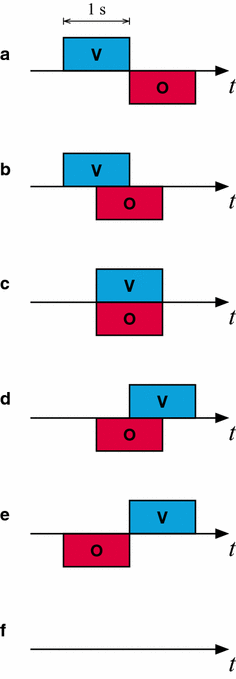
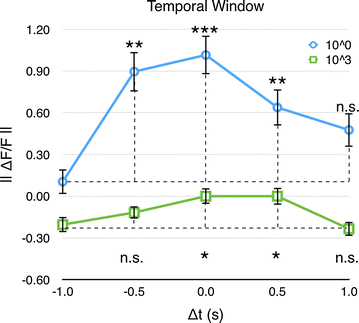
Results: We investigated the responses to multimodal stimuli consisting of an odour and a colour in the antennal lobe and mushroom body of the moth Manduca sexta. The mushroom body shows enhanced responses for multimodal stimuli consisting of a general flower odour and a blue colour. No such effect was seen for a bergamot odour. The enhancement shows an inverse effectiveness where the responses to weaker multimodal stimuli are amplified more than those to stronger stimuli. Furthermore, the enhancement depends on the precise timing of the two stimulus components.
Conclusions: Insect multimodal processing show both the principle of inverse effectiveness and the existence of an optimal temporal window.
Keywords: Inverse effeciveness; Moth; Multmodal interaction; Superadditivity; Temporal window.
Figures




References
-
- Stein BE, Meredith MA. The merging of the senses. Cambridge: The MIT Press; 1993.
-
- Eagleman DM. How does the timing of neural signals map onto the timing of perception? In: Space and time in perception and action. 2010. p. 216–231.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

