Castration-Resistant Prostate Cancer Tissue Acquisition From Bone Metastases for Molecular Analyses
- PMID: 27246360
- PMCID: PMC5132155
- DOI: 10.1016/j.clgc.2016.04.016
Castration-Resistant Prostate Cancer Tissue Acquisition From Bone Metastases for Molecular Analyses
Abstract
Background: The urgent need for castration-resistant prostate cancer molecular characterization to guide treatment has been constrained by the disease's predilection to metastasize primarily to bone. We hypothesized that the use of clinical and imaging criteria could maximize tissue acquisition from bone marrow biopsies (BMBs). We aimed to develop a score for the selection of patients undergoing BMB.
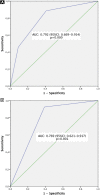
Materials and methods: A total of 115 BMBs were performed in 101 patients: 57 were included in a derivation set and 58 were used as the validation set. The clinical and laboratory data and prebiopsy computed tomography parameters (Hounsfield units [HUs]) were determined. A score for the prediction of biopsy positivity was developed from logistic regression analysis of the derivation set and tested in the validation set.
Results: Of the 115 biopsy specimens, 75 (62.5%) were positive; 35 (61.4%) in the test set and 40 (69%) in the validation set. On univariable analysis, hemoglobin (P = .019), lactate dehydrogenase (P = .003), prostate-specific antigen (P = .005), and mean HUs (P = .004) were selected. A score based on the LDH level (≥ 225 IU/L) and mean HUs (≥ 125) was developed in multivariate analysis and was associated with BMB positivity in the validation set (odds ratio, 5.1; 95% confidence interval, 1.9%-13.4%; P = .001). The area under the curve of the score was 0.79 in the test set and 0.77 in the validation set.
Conclusion: BMB of the iliac crest is a feasible technique for obtaining tumor tissue for genomic analysis in patients with castration-resistant prostate cancer metastatic to the bone. A signature based on the mean HUs and LDH level can predict a positive yield with acceptable internal validity. Prospective studies of independent cohorts are needed to establish the external validity of the score.
Keywords: Biopsy; Bone marrow; Computed tomography; Hounsfield units; Molecular biology.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Ferlay J., Soerjomataram I., Ervik M. International Agency for Research on Cancer; Lyon: 2013. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11.http://globocan.iarc.fr Available at: Accessed April 13, 2014.
-
- Yap T.A., Sandhu S.K., Workman P., de Bono J.S. Envisioning the future of early anticancer drug development. Nat Rev Cancer. 2010;10:514–523. - PubMed
-
- de Bono J.S., Ashworth A. Translating cancer research into targeted therapeutics. Nature. 2010;467:543–549. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous