Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy
- PMID: 27246539
- PMCID: PMC4972679
- DOI: 10.1158/2159-8290.CD-15-1177
Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy
Abstract
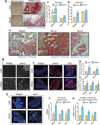
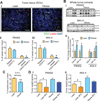
It remains unclear how obesity worsens treatment outcomes in patients with pancreatic ductal adenocarcinoma (PDAC). In normal pancreas, obesity promotes inflammation and fibrosis. We found in mouse models of PDAC that obesity also promotes desmoplasia associated with accelerated tumor growth and impaired delivery/efficacy of chemotherapeutics through reduced perfusion. Genetic and pharmacologic inhibition of angiotensin-II type-1 receptor reverses obesity-augmented desmoplasia and tumor growth and improves response to chemotherapy. Augmented activation of pancreatic stellate cells (PSC) in obesity is induced by tumor-associated neutrophils (TAN) recruited by adipocyte-secreted IL1β. PSCs further secrete IL1β, and inactivation of PSCs reduces IL1β expression and TAN recruitment. Furthermore, depletion of TANs, IL1β inhibition, or inactivation of PSCs prevents obesity-accelerated tumor growth. In patients with pancreatic cancer, we confirmed that obesity is associated with increased desmoplasia and reduced response to chemotherapy. We conclude that cross-talk between adipocytes, TANs, and PSCs exacerbates desmoplasia and promotes tumor progression in obesity.
Significance: Considering the current obesity pandemic, unraveling the mechanisms underlying obesity-induced cancer progression is an urgent need. We found that the aggravation of desmoplasia is a key mechanism of obesity-promoted PDAC progression. Importantly, we discovered that clinically available antifibrotic/inflammatory agents can improve the treatment response of PDAC in obese hosts. Cancer Discov; 6(8); 852-69. ©2016 AACR.See related commentary by Bronte and Tortora, p. 821This article is highlighted in the In This Issue feature, p. 803.
©2016 American Association for Cancer Research.
Figures
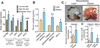




Comment in
-
Adipocytes and Neutrophils Give a Helping Hand to Pancreatic Cancers.Cancer Discov. 2016 Aug;6(8):821-3. doi: 10.1158/2159-8290.CD-16-0682. Cancer Discov. 2016. PMID: 27485002
-
Cancer: Fat and the fate of pancreatic tumours.Nature. 2016 Aug 11;536(7615):157-8. doi: 10.1038/nature19419. Epub 2016 Aug 3. Nature. 2016. PMID: 27487208 No abstract available.
References
-
- American Cancer Society: Cancer Facts and Figures. 2015
-
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

