Rotation of Vibrio fischeri Flagella Produces Outer Membrane Vesicles That Induce Host Development
- PMID: 27246572
- PMCID: PMC4966437
- DOI: 10.1128/JB.00101-16
Rotation of Vibrio fischeri Flagella Produces Outer Membrane Vesicles That Induce Host Development
Abstract
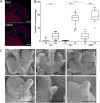
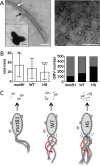
Using the squid-vibrio association, we aimed to characterize the mechanism through which Vibrio fischeri cells signal morphogenesis of the symbiotic light-emitting organ. The symbiont releases two cell envelope molecules, peptidoglycan (PG) and lipopolysaccharide (LPS) that, within 12 h of light organ colonization, act in synergy to trigger normal tissue development. Recent work has shown that outer membrane vesicles (OMVs) produced by V. fischeri are sufficient to induce PG-dependent morphogenesis; however, the mechanism(s) of OMV release by these bacteria has not been described. Like several genera of both beneficial and pathogenic bacteria, V. fischeri cells elaborate polar flagella that are enclosed by an extension of the outer membrane, whose function remains unclear. Here, we present evidence that along with the well-recognized phenomenon of blebbing from the cell's surface, rotation of this sheathed flagellum also results in the release of OMVs. In addition, we demonstrate that most of the development-inducing LPS is associated with these OMVs and that the presence of the outer membrane protein OmpU but not the LPS O antigen on these OMVs is important in triggering normal host development. These results also present insights into a possible new mechanism of LPS release by pathogens with sheathed flagella.
Importance: Determining the function(s) of sheathed flagella in bacteria has been challenging, because no known mutation results only in the loss of this outer membrane-derived casing. Nevertheless, the presence of a sheathed flagellum in such host-associated genera as Vibrio, Helicobacter, and Brucella has led to several proposed functions, including physical protection of the flagella and masking of their immunogenic flagellins. Using the squid-vibrio light organ symbiosis, we demonstrate another role, that of V. fischeri cells require rotating flagella to induce apoptotic cell death within surface epithelium, which is a normal step in the organ's development. Further, we present evidence that this rotation releases apoptosis-triggering lipopolysaccharide in the form of outer membrane vesicles. Such release may also occur by pathogens but with different outcomes for the host.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


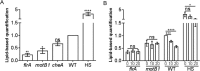


References
-
- Kremer N, Philipp EER, Carpentier M-C, Brennan CA, Kraemer L, Altura MA, Augustin R, Häsler R, Heath-Heckman EAC, Peyer SM, Schwartzman J, Rader BA, Ruby EG, Rosenstiel P, McFall-Ngai MJ. 2013. Initial symbiont contact orchestrates host-organ-wide transcriptional changes that prime tissue colonization. Cell Host Microbe 14:183–194. doi: 10.1016/j.chom.2013.07.006. - DOI - PMC - PubMed
-
- Montgomery MK, McFall-Ngai M. 1994. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development 120:1719–1729. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

