Development-on-chip: in vitro neural tube patterning with a microfluidic device
- PMID: 27246712
- PMCID: PMC4920155
- DOI: 10.1242/dev.126847
Development-on-chip: in vitro neural tube patterning with a microfluidic device
Abstract
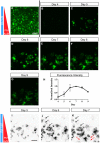
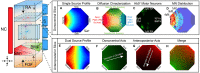
Embryogenesis is a highly regulated process in which the precise spatial and temporal release of soluble cues directs differentiation of multipotent stem cells into discrete populations of specialized adult cell types. In the spinal cord, neural progenitor cells are directed to differentiate into adult neurons through the action of mediators released from nearby organizing centers, such as the floor plate and paraxial mesoderm. These signals combine to create spatiotemporal diffusional landscapes that precisely regulate the development of the central nervous system (CNS). Currently, in vivo and ex vivo studies of these signaling factors present some inherent ambiguity. In vitro methods are preferred for their enhanced experimental clarity but often lack the technical sophistication required for biological realism. In this article, we present a versatile microfluidic platform capable of mimicking the spatial and temporal chemical environments found in vivo during neural tube development. Simultaneous opposing and/or orthogonal gradients of developmental morphogens can be maintained, resulting in neural tube patterning analogous to that observed in vivo.
Keywords: Differentiation; Microfluidic; Mouse; Neuron; Patterning; Stem cell.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures




References
-
- Amadi O. C., Steinhauser M. L., Nishi Y., Chung S., Kamm R. D., McMahon A. P. and Lee R. T. (2010). A low resistance microfluidic system for the creation of stable concentration gradients in a defined 3D microenvironment. Biomed. Microdevices 12, 1027-1041. 10.1007/s10544-010-9457-7 - DOI - PMC - PubMed
-
- Bushati N. and Briscoe J. (1994). Regulation of neuronal subtype identity in the vertebrate neural tube (neuronal subtype identity regulation). eLS.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

