Cathepsin A inhibition attenuates myocardial infarction-induced heart failure on the functional and proteomic levels
- PMID: 27246731
- PMCID: PMC4888645
- DOI: 10.1186/s12967-016-0907-8
Cathepsin A inhibition attenuates myocardial infarction-induced heart failure on the functional and proteomic levels
Abstract
Background: Myocardial infarction (MI) is a major cause of heart failure. The carboxypeptidase cathepsin A is a novel target in the treatment of cardiac failure. We aim to show that recently developed inhibitors of the protease cathepsin A attenuate post-MI heart failure.
Methods: Mice were subjected to permanent left anterior descending artery (LAD) ligation or sham operation. 24 h post-surgery, LAD-ligated animals were treated with daily doses of the cathepsin A inhibitor SAR1 or placebo. After 4 weeks, the three groups (sham, MI-placebo, MI-SAR1) were evaluated.
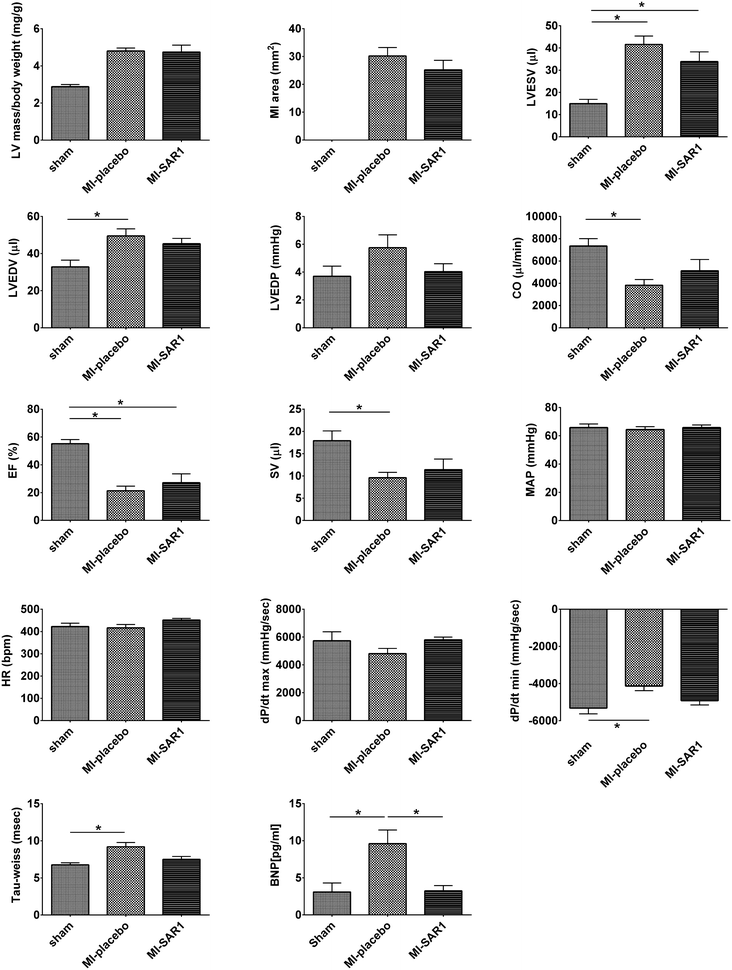
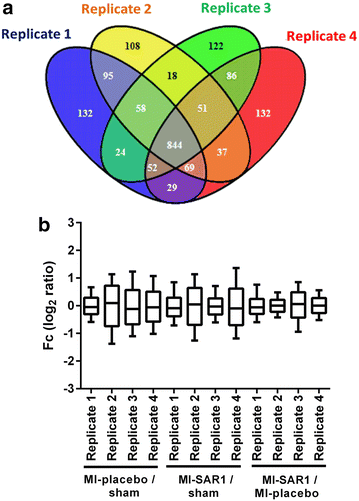
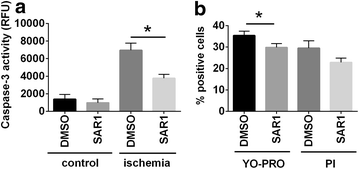
Results: Compared to sham-operated animals, placebo-treated mice showed significantly impaired cardiac function and increased plasma BNP levels. Cathepsin A inhibition prevented the increase of plasma BNP levels and displayed a trend towards improved cardiac functionality. Proteomic profiling was performed for the three groups (sham, MI-placebo, MI-SAR1). More than 100 proteins were significantly altered in placebo-treated LAD ligation compared to the sham operation, including known markers of cardiac failure as well as extracellular/matricellular proteins. This ensemble constitutes a proteome fingerprint of myocardial infarction induced by LAD ligation in mice. Cathepsin A inhibitor treatment normalized the marked increase of the muscle stress marker CA3 as well as of Igγ 2b and fatty acid synthase. For numerous further proteins, cathepsin A inhibition partially dampened the LAD ligation-induced proteome alterations.
Conclusions: Our proteomic and functional data suggest that cathepsin A inhibition has cardioprotective properties and support a beneficial effect of cathepsin A inhibition in the treatment of heart failure after myocardial infarction.
Keywords: Cardiovascular diseases; Drug therapy; Heart failure; Mouse model; Myocardial infarction.
Figures



References
-
- Seyrantepe V, Hinek A, Peng J, Fedjaev M, Ernest S, Kadota Y, et al. Enzymatic activity of lysosomal carboxypeptidase (cathepsin) A is required for proper elastic fiber formation and inactivation of endothelin-1. Circulation. 2008;117:1973–1981. doi: 10.1161/CIRCULATIONAHA.107.733212. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

