Protein-Protein Interactions, Not Substrate Recognition, Dominate the Turnover of Chimeric Assembly Line Polyketide Synthases
- PMID: 27246853
- PMCID: PMC4965586
- DOI: 10.1074/jbc.M116.730531
Protein-Protein Interactions, Not Substrate Recognition, Dominate the Turnover of Chimeric Assembly Line Polyketide Synthases
Abstract
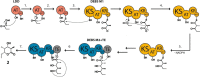
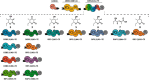
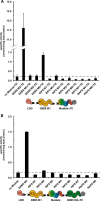
The potential for recombining intact polyketide synthase (PKS) modules has been extensively explored. Both enzyme-substrate and protein-protein interactions influence chimeric PKS activity, but their relative contributions are unclear. We now address this issue by studying a library of 11 bimodular and 8 trimodular chimeric PKSs harboring modules from the erythromycin, rifamycin, and rapamycin synthases. Although many chimeras yielded detectable products, nearly all had specific activities below 10% of the reference natural PKSs. Analysis of selected bimodular chimeras, each with the same upstream module, revealed that turnover correlated with the efficiency of intermodular chain translocation. Mutation of the acyl carrier protein (ACP) domain of the upstream module in one chimera at a residue predicted to influence ketosynthase-ACP recognition led to improved turnover. In contrast, replacement of the ketoreductase domain of the upstream module by a paralog that produced the enantiomeric ACP-bound diketide caused no changes in processing rates for each of six heterologous downstream modules compared with those of the native diketide. Taken together, these results demonstrate that protein-protein interactions play a larger role than enzyme-substrate recognition in the evolution or design of catalytically efficient chimeric PKSs.
Keywords: enzyme turnover; polyketide; protein chimera; protein engineering; protein-protein interaction.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Walsh C. (2004) Polyketide and nonribosomal peptide antibiotics: modularity and versatility. Science 303, 1805–1810 - PubMed
-
- Khosla C., Tang Y., Chen A. Y., Schnarr N. A., and Cane D. E. (2007) Structure and mechanism of the 6-deoxyerythronolide B synthase. Annu. Rev. Biochem. 76, 195–221 - PubMed
-
- Cortes J., Haydock S. F., Roberts G. A., Bevitt D. J., and Leadlay P. F. (1990) An unusually large multifunctional polypeptide in the erythromycin-producing polyketide synthase of Saccharopolyspora erythraea. Nature 348, 176–178 - PubMed
-
- Donadio S., Staver M. J., McAlpine J. B., Swanson S. J., and Katz L. (1991) Modular organization of genes required for complex polyketide biosynthesis. Science 252, 675–679 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

