The CaMKII/GluN2B Protein Interaction Maintains Synaptic Strength
- PMID: 27246855
- PMCID: PMC4965558
- DOI: 10.1074/jbc.M116.734822
The CaMKII/GluN2B Protein Interaction Maintains Synaptic Strength
Abstract
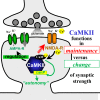
Learning, memory, and cognition are thought to require normal long-term potentiation (LTP) of synaptic strength, which in turn requires binding of the Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) to the NMDA-type glutamate receptor (NMDAR) subunit GluN2B. For LTP induction, many additional required players are known. Here we tested the hypothesis that CaMKII/GluN2B binding also mediates the more elusive maintenance of synaptic strength. Intriguingly, the CaMKII inhibitor tatCN21 reduces synaptic strength only at high concentrations necessary for CaMKII/NMDAR disruption (20 μm) but not at lower concentrations sufficient for kinase inhibition (5 μm). However, increased concentration also causes unrelated effects. Thus, to distinguish between correlation and causality, we used a pharmacogenetic approach. In a mouse with a mutant NMDAR GluN2B subunit that is CaMKII binding-incompetent, any tatCN21 effects that are specific to the CaMKII/GluN2B interaction should be abolished, and any remaining tatCN21 effects have to be nonspecific (i.e. mediated by other targets). The results showed that the persistent reduction of synaptic strength by transient application of 20 μm tatCN21 had a nonspecific presynaptic component (on fiber volley amplitude) that was unrelated to the CaMKII/GluN2B interaction or CaMKII activity. However, the remaining component of the persistent tatCN21 effect was almost completely abolished in the GluN2B mutant mouse. These results highlight the requirement for stringent pharmacogenetic approaches to separate specific on-target effects from nonspecific off-target effects. Importantly, they also demonstrate that the CaMKII/GluN2B interaction is required not only for normal LTP induction but also for the maintenance of synaptic strength.
Keywords: Ca2+/calmodulin-dependent protein kinase II (CaMKII); N-methyl-d-aspartate receptor (NMDA receptor, NMDAR); glutamate receptor; hippocampus; synapse.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Shonesy B. C., Jalan-Sakrikar N., Cavener V. S., and Colbran R. J. (2014) CaMKII: a molecular substrate for synaptic plasticity and memory. Prog. Mol. Biol. Transl. Sci. 122, 61–87 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

