The importance of collecting structured clinical information on multiple sclerosis
- PMID: 27246898
- PMCID: PMC4888646
- DOI: 10.1186/s12916-016-0627-1
The importance of collecting structured clinical information on multiple sclerosis
Abstract
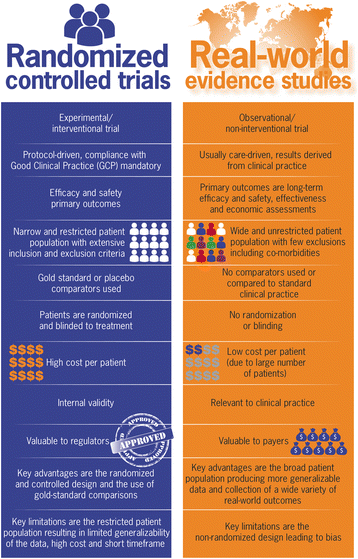
Background: Randomized controlled trials (RCTs) are the 'gold standard' in the generation of drug efficacy and safety evidence. However, enrolment criteria, timelines and atypical comparators of RCTs limit their relevance to standard clinical practice.
Discussion: Real-world data (RWD) provide longitudinal information on the comparative effectiveness and tolerability of drugs, as well as their impact on resource use, medical costs, and pharmacoeconomic and patient-reported outcomes. This is particularly important in multiple sclerosis (MS), where economic treatment benefits of long-term disability reduction are a cornerstone of payer drug approvals - these are typically not examined in the RCT itself but modelled using real-world datasets. Importantly, surrogate markers used in RCTs to predict the prevention of long-term disability progression can only truly be assessed through RWD methodologies. We discuss the differences between RCTs and RWD studies, describe how RWD complements the evidence base from RCTs in MS, summarize the different methods of RWD collection, and explain the importance of structuring data analysis to avoid bias. Guidance on performing and identifying high-quality real-world evidence studies is also provided.
Keywords: Multiple sclerosis; Pharmacoeconomics; Randomised controlled trials; Real-world data; Real-world evidence; Registries.
Figures
References
-
- Milnes F, Bergvall N, Olson M, Capkun-Niggli G, Bonzani I, Makin C. Use of real-world data to assess outcomes in patients with multiple sclerosis in a real-life clinical setting. 10th Annual Meeting of Health Technology Assessment international (HTAi). Seoul: Poster P1082; 2013.
-
- Association of the British Pharmaceutical Industry. Demonstrating value with real world data: a practical guide. 2011. http://www.abpi.org.uk/our-work/library/guidelines/Pages/real-world-data.... Accessed 25 February 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

