Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity
- PMID: 27247136
- PMCID: PMC4946200
- DOI: 10.1182/blood-2016-03-707133
Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity
Abstract
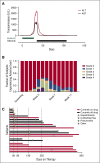
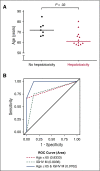
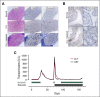
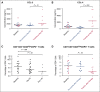
Idelalisib is a small-molecule inhibitor of PI3Kδ with demonstrated efficacy for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL). To evaluate idelalisib as front-line therapy, we enrolled 24 subjects in a phase 2 study consisting of 2 months of idelalisib monotherapy followed by 6 months of combination therapy with idelalisib and the anti-CD20 antibody ofatumumab. After a median follow-up period of 14.7 months, hepatotoxicity was found to be a frequent and often severe adverse event. A total of 19 subjects (79%) experienced either grade ≥1 ALT or AST elevation during the study, and 13 subjects (54%) experienced grade ≥3 transaminitis. The median time to development of transaminitis was 28 days, occurring before ofatumumab introduction. Younger age and mutated immunoglobulin heavy chain status were significant risk factors for the development of hepatotoxicity. Multiple lines of evidence suggest that this hepatotoxicity was immune mediated. A lymphocytic infiltrate was seen on liver biopsy specimens taken from 2 subjects with transaminitis, and levels of the proinflammatory cytokines CCL-3 and CCL-4 were higher in subjects experiencing hepatotoxicity. All cases of transaminitis resolved either by holding the drug, initiating immunosuppressants, or both, and rates of recurrent toxicity were lower in patients taking steroids when idelalisib was reinitiated. A decrease in peripheral blood regulatory T cells was seen in patients experiencing toxicity on therapy, which is consistent with an immune-mediated mechanism. These results suggest that caution should be taken as drugs within this class are developed for CLL, particularly in younger patients who have not received prior disease-specific therapy. This study was registered at www.clinicaltrials.gov as #NCT02135133.
© 2016 by The American Society of Hematology.
Figures
References
-
- Sharman JP, Coutre SE, Furman RR, et al. Second interim analysis of a phase 3 study of idelalisib (ZYDELIG®) plus rituximab (R) for relapsed chronic lymphocytic leukemia (CLL): Efficacy analysis in patient subpopulations with del(17p) and other adverse prognostic factors [abstract]. Blood. 2014;124(21) Abstract 330.
-
- Coutre SE, Leonard JP, Furman RR, et al. Update on a phase 1 study of the selective PI3K-delta inhibitor, idelalisib (GS-1101) in combination with ofatumumab in patients with relapsed or refractory chronic lymphocytic leukemia. Haematologica. 2013;98(suppl 1):1.
-
- De Vos S, Leonard JP, Barrientos JC, et al. A phase 1 study of the selective PI3Kδ inhibitor idelalisib (GS-1101) in combination with therapeutic anti-CD20 antibodies (rituximab or ofatumumab) in patients with relapsed or refractory chronic lymphocytic leukemia [abstract]. Blood. 2013;122(21) Abstract 4180.
-
- Coutre S, Barrientos JC, Brown JR, et al. Safety of idelalisib in B-cell malignancies: Integrated analysis of eight clinical trials. ASCO Meeting Abstracts. 2015;33(15_suppl):e18030.
-
- Zelenetz AD, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib monotherapy in previously untreated patients ≥65 years with chronic lymphocytic leukemia or small lymphocytic lymphoma. Paper presented at the Annual Meeting of the American Society of Hematology. December 6, 2014. San Francisco, CA.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

