miR-1 suppresses the growth of esophageal squamous cell carcinoma in vivo and in vitro through the downregulation of MET, cyclin D1 and CDK4 expression
- PMID: 27247259
- PMCID: PMC4899011
- DOI: 10.3892/ijmm.2016.2619
miR-1 suppresses the growth of esophageal squamous cell carcinoma in vivo and in vitro through the downregulation of MET, cyclin D1 and CDK4 expression
Abstract
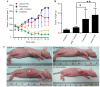
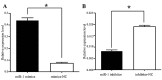
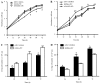
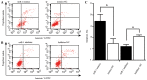
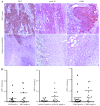
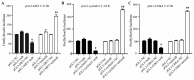
Several aberrant microRNAs (miRNAs or miRs) have been implicated in esophageal cancer (EC), which is widely prevalent in China. However, their role in EC tumorigenesis has not yet been fully elucidated. In the present study, we determined that miR‑1 was downregulated in esophageal squamous cell carcinoma (ESCC) tissues compared with adjacent non-neoplastic tissues using RT-qPCR, and confirmed this using an ESCC cell line. Using a nude mouse xenograft model, we confirmed that the re-expression of miR‑1 significantly inhibited ESCC tumor growth. A tetrazolium assay and a trypan blue exclusion assay revealed that miR‑1 suppressed ESCC cell proliferation and increased apoptosis, whereas the silencing of miR‑1 promoted cell proliferation and decreased apoptosis, suggesting that miR‑1 is a novel tumor suppressor. To elucidate the molecular mechanisms of action of miR‑1 in ESCC, we investigated putative targets using bioinformatics tools. MET, cyclin D1 and cyclin-dependent kinase 4 (CDK4), which are involved in the hepatocyte growth factor (HGF)/MET signaling pathway, were found to be targets of miR‑1. miR‑1 expression inversely correlated with MET, cyclin D1 and CDK4 expression in ESCC cells. miR‑1 directly targeted MET, cyclin D1 and CDK4, suppressing ESCC cell growth. The newly identified miR‑1/MET/cyclin D1/CDK4 axis provides new insight into the molecular mechanisms of ESCC pathogenesis and indicates a novel strategy for the diagnosis and treatment of ESCC.
Figures


Similar articles
-
MiR-214 promotes cell meastasis and inhibites apoptosis of esophageal squamous cell carcinoma via PI3K/AKT/mTOR signaling pathway.Biomed Pharmacother. 2018 Sep;105:350-361. doi: 10.1016/j.biopha.2018.05.149. Epub 2018 Jun 1. Biomed Pharmacother. 2018. PMID: 29864623
-
Downregulation of microRNA‑449a‑5p promotes esophageal squamous cell carcinoma cell proliferation via cyclin D1 regulation.Mol Med Rep. 2018 Jul;18(1):848-854. doi: 10.3892/mmr.2018.9030. Epub 2018 May 17. Mol Med Rep. 2018. PMID: 29845226 Free PMC article.
-
Downregulation of miR-503 Promotes ESCC Cell Proliferation, Migration, and Invasion by Targeting Cyclin D1.Genomics Proteomics Bioinformatics. 2017 Jun;15(3):208-217. doi: 10.1016/j.gpb.2017.04.003. Epub 2017 Jun 9. Genomics Proteomics Bioinformatics. 2017. PMID: 28602785 Free PMC article.
-
Molecular aspects of esophageal squamous cell carcinoma carcinogenesis.Arq Gastroenterol. 2003 Oct-Dec;40(4):256-61. doi: 10.1590/s0004-28032003000400011. Epub 2004 May 31. Arq Gastroenterol. 2003. PMID: 15264049 Review.
-
Cyclin D1, cancer progression, and opportunities in cancer treatment.J Mol Med (Berl). 2016 Dec;94(12):1313-1326. doi: 10.1007/s00109-016-1475-3. Epub 2016 Oct 2. J Mol Med (Berl). 2016. PMID: 27695879 Free PMC article. Review.
Cited by
-
Retracted Article: Long noncoding RNA PCA3 regulates glycolysis, viability and apoptosis by mediating the miR-1/CDK4 axis in prostate cancer.RSC Adv. 2018 Nov 8;8(66):37564-37572. doi: 10.1039/c8ra08083f. eCollection 2018 Nov 7. RSC Adv. 2018. Retraction in: RSC Adv. 2021 Feb 2;11(11):5894. doi: 10.1039/d1ra90066h. PMID: 35558606 Free PMC article. Retracted.
-
MicroRNA-1 suppresses proliferation, migration and invasion by targeting Notch2 in esophageal squamous cell carcinoma.Sci Rep. 2018 Mar 26;8(1):5183. doi: 10.1038/s41598-018-23421-3. Sci Rep. 2018. PMID: 29581534 Free PMC article.
-
A review on the role of cyclin dependent kinases in cancers.Cancer Cell Int. 2022 Oct 20;22(1):325. doi: 10.1186/s12935-022-02747-z. Cancer Cell Int. 2022. PMID: 36266723 Free PMC article. Review.
-
Tumor suppressor miR-1 inhibits tumor growth and metastasis by simultaneously targeting multiple genes.Oncotarget. 2017 Jun 27;8(26):42043-42060. doi: 10.18632/oncotarget.14927. Oncotarget. 2017. PMID: 28159933 Free PMC article.
-
The PI3K/mTOR dual inhibitor BEZ235 suppresses proliferation and migration and reverses multidrug resistance in acute myeloid leukemia.Acta Pharmacol Sin. 2017 Mar;38(3):382-391. doi: 10.1038/aps.2016.121. Epub 2017 Jan 2. Acta Pharmacol Sin. 2017. PMID: 28042875 Free PMC article.
References
-
- Layke JC, Lopez PP. Esophageal cancer: a review and update. Am Fam Physician. 2006;73:2187–2194. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

