ZDHHC3 Tyrosine Phosphorylation Regulates Neural Cell Adhesion Molecule Palmitoylation
- PMID: 27247265
- PMCID: PMC4985929
- DOI: 10.1128/MCB.00144-16
ZDHHC3 Tyrosine Phosphorylation Regulates Neural Cell Adhesion Molecule Palmitoylation
Abstract
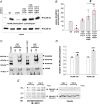
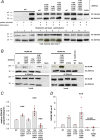
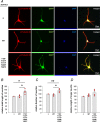
The neural cell adhesion molecule (NCAM) mediates cell-cell and cell-matrix adhesion. It is broadly expressed in the nervous system and regulates neurite outgrowth, synaptogenesis, and synaptic plasticity. Previous in vitro studies revealed that palmitoylation of NCAM is required for fibroblast growth factor 2 (FGF2)-stimulated neurite outgrowth and identified the zinc finger DHHC (Asp-His-His-Cys)-containing proteins ZDHHC3 and ZDHHC7 as specific NCAM-palmitoylating enzymes. Here, we verified that FGF2 controlled NCAM palmitoylation in vivo and investigated molecular mechanisms regulating NCAM palmitoylation by ZDHHC3. Experiments with overexpression and pharmacological inhibition of FGF receptor (FGFR) and Src revealed that these kinases control tyrosine phosphorylation of ZDHHC3 and that ZDHHC3 is phosphorylated by endogenously expressed FGFR and Src proteins. By site-directed mutagenesis, we found that Tyr18 is an FGFR1-specific ZDHHC3 phosphorylation site, while Tyr295 and Tyr297 are specifically phosphorylated by Src kinase in cell-based and cell-free assays. Abrogation of tyrosine phosphorylation increased ZDHHC3 autopalmitoylation, enhanced interaction with NCAM, and upregulated NCAM palmitoylation. Expression of ZDHHC3 with tyrosine mutated in cultured hippocampal neurons promoted neurite outgrowth. Our findings for the first time highlight that FGFR- and Src-mediated tyrosine phosphorylation of ZDHHC3 modulates ZDHHC3 enzymatic activity and plays a role in neuronal morphogenesis.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
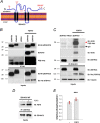
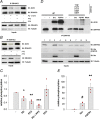
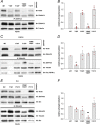
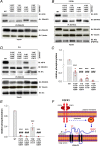

Similar articles
-
ShcA regulates neurite outgrowth stimulated by neural cell adhesion molecule but not by fibroblast growth factor 2: evidence for a distinct fibroblast growth factor receptor response to neural cell adhesion molecule activation.J Neurochem. 2004 Nov;91(3):694-703. doi: 10.1111/j.1471-4159.2004.02772.x. J Neurochem. 2004. PMID: 15485499
-
Fibroblast growth factor-regulated palmitoylation of the neural cell adhesion molecule determines neuronal morphogenesis.J Neurosci. 2008 Sep 3;28(36):8897-907. doi: 10.1523/JNEUROSCI.2171-08.2008. J Neurosci. 2008. PMID: 18768683 Free PMC article.
-
An NCAM-derived FGF-receptor agonist, the FGL-peptide, induces neurite outgrowth and neuronal survival in primary rat neurons.J Neurochem. 2004 Nov;91(4):920-35. doi: 10.1111/j.1471-4159.2004.02779.x. J Neurochem. 2004. PMID: 15525346
-
Signaling mechanisms of neurite outgrowth induced by the cell adhesion molecules NCAM and N-cadherin.Cell Mol Life Sci. 2008 Nov;65(23):3809-21. doi: 10.1007/s00018-008-8290-0. Cell Mol Life Sci. 2008. PMID: 18791849 Free PMC article. Review.
-
Structural biology of NCAM homophilic binding and activation of FGFR.J Neurochem. 2005 Sep;94(5):1169-79. doi: 10.1111/j.1471-4159.2005.03284.x. Epub 2005 Jul 25. J Neurochem. 2005. PMID: 16045455 Review.
Cited by
-
S-acylated Golga7b stabilises DHHC5 at the plasma membrane to regulate cell adhesion.EMBO Rep. 2019 Oct 4;20(10):e47472. doi: 10.15252/embr.201847472. Epub 2019 Aug 12. EMBO Rep. 2019. PMID: 31402609 Free PMC article.
-
Attenuated palmitoylation of serotonin receptor 5-HT1A affects receptor function and contributes to depression-like behaviors.Nat Commun. 2019 Sep 2;10(1):3924. doi: 10.1038/s41467-019-11876-5. Nat Commun. 2019. PMID: 31477731 Free PMC article.
-
Protein palmitoylation and cancer.EMBO Rep. 2018 Oct;19(10):e46666. doi: 10.15252/embr.201846666. Epub 2018 Sep 19. EMBO Rep. 2018. PMID: 30232163 Free PMC article. Review.
-
S-Palmitoylation of the sodium channel Nav1.6 regulates its activity and neuronal excitability.J Biol Chem. 2020 May 1;295(18):6151-6164. doi: 10.1074/jbc.RA119.012423. Epub 2020 Mar 11. J Biol Chem. 2020. PMID: 32161114 Free PMC article.
-
The expression of select genes necessary for membrane-associated estrogen receptor signaling differ by sex in adult rat hippocampus.Steroids. 2019 Feb;142:21-27. doi: 10.1016/j.steroids.2017.09.012. Epub 2017 Sep 28. Steroids. 2019. PMID: 28962849 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
