The adenovirus E4-ORF3 protein functions as a SUMO E3 ligase for TIF-1γ sumoylation and poly-SUMO chain elongation
- PMID: 27247387
- PMCID: PMC4914182
- DOI: 10.1073/pnas.1603872113
The adenovirus E4-ORF3 protein functions as a SUMO E3 ligase for TIF-1γ sumoylation and poly-SUMO chain elongation
Abstract
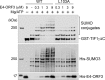
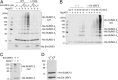
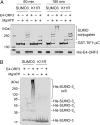
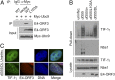
The adenovirus (Ad) early region 4 (E4)-ORF3 protein regulates diverse cellular processes to optimize the host environment for the establishment of Ad replication. E4-ORF3 self-assembles into multimers to form a nuclear scaffold in infected cells and creates distinct binding interfaces for different cellular target proteins. Previous studies have shown that the Ad5 E4-ORF3 protein induces sumoylation of multiple cellular proteins and subsequent proteasomal degradation of some of them, but the detailed mechanism of E4-ORF3 function remained unknown. Here, we investigate the role of E4-ORF3 in the sumoylation process by using transcription intermediary factor (TIF)-1γ as a substrate. Remarkably, we discovered that purified E4-ORF3 protein stimulates TIF-1γ sumoylation in vitro, demonstrating that E4-ORF3 acts as a small ubiquitin-like modifier (SUMO) E3 ligase. Furthermore, E4-ORF3 significantly increases poly-SUMO3 chain formation in vitro in the absence of substrate, showing that E4-ORF3 has SUMO E4 elongase activity. An E4-ORF3 mutant, which is defective in protein multimerization, exhibited severely decreased activity, demonstrating that E4-ORF3 self-assembly is required for these activities. Using a SUMO3 mutant, K11R, we found that E4-ORF3 facilitates the initial acceptor SUMO3 conjugation to TIF-1γ as well as poly-SUMO chain elongation. The E4-ORF3 protein displays no SUMO-targeted ubiquitin ligase activity in our assay system. These studies reveal the mechanism by which E4-ORF3 targets specific cellular proteins for sumoylation and proteasomal degradation and provide significant insight into how a small viral protein can play a role as a SUMO E3 ligase and E4-like SUMO elongase to impact a variety of cellular responses.
Keywords: E3 ligase; SUMO; TIF-1γ; adenovirus; proteasome degradation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Mechanism of Adenovirus E4-ORF3-Mediated SUMO Modifications.mBio. 2019 Feb 26;10(1):e00022-19. doi: 10.1128/mBio.00022-19. mBio. 2019. PMID: 30808699 Free PMC article.
-
Adenovirus E4-ORF3 Targets PIAS3 and Together with E1B-55K Remodels SUMO Interactions in the Nucleus and at Virus Genome Replication Domains.J Virol. 2015 Oct;89(20):10260-72. doi: 10.1128/JVI.01091-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223632 Free PMC article.
-
The Adenovirus E4-ORF3 Protein Stimulates SUMOylation of General Transcription Factor TFII-I to Direct Proteasomal Degradation.mBio. 2016 Jan 26;7(1):e02184-15. doi: 10.1128/mBio.02184-15. mBio. 2016. PMID: 26814176 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator degradation: cross-talk between the ubiquitylation and SUMOylation pathways.FEBS J. 2013 Sep;280(18):4430-8. doi: 10.1111/febs.12415. Epub 2013 Jul 22. FEBS J. 2013. PMID: 23809253 Free PMC article. Review.
-
[Ubiquitins, proteasomes, sumoylation and application today and in future for cancer and other diseases therapy II. Sumoylation and neddylation as posttranslational modifications of proteins and their ubiquitinylation and its significance].Vnitr Lek. 2006 Jun;52(6):619-27. Vnitr Lek. 2006. PMID: 16871767 Review. Czech.
Cited by
-
Contribution of Epstein-Barr Virus Lytic Proteins to Cancer Hallmarks and Implications from Other Oncoviruses.Cancers (Basel). 2023 Apr 2;15(7):2120. doi: 10.3390/cancers15072120. Cancers (Basel). 2023. PMID: 37046781 Free PMC article. Review.
-
The interactions between PML nuclear bodies and small and medium size DNA viruses.Virol J. 2023 May 1;20(1):82. doi: 10.1186/s12985-023-02049-4. Virol J. 2023. PMID: 37127643 Free PMC article. Review.
-
Adenoviral strategies to overcome innate cellular responses to infection.FEBS Lett. 2019 Dec;593(24):3484-3495. doi: 10.1002/1873-3468.13680. Epub 2019 Nov 26. FEBS Lett. 2019. PMID: 31721176 Free PMC article. Review.
-
The Role of SUMO E3 Ligases in Signaling Pathway of Cancer Cells.Int J Mol Sci. 2022 Mar 26;23(7):3639. doi: 10.3390/ijms23073639. Int J Mol Sci. 2022. PMID: 35408996 Free PMC article. Review.
-
ATO (Arsenic Trioxide) Effects on Promyelocytic Leukemia Nuclear Bodies Reveals Antiviral Intervention Capacity.Adv Sci (Weinh). 2020 Feb 27;7(8):1902130. doi: 10.1002/advs.201902130. eCollection 2020 Apr. Adv Sci (Weinh). 2020. PMID: 32328411 Free PMC article.
References
-
- Berk A. Fields Virology. Lippincott Williams & Wilkins; Philadelphia, PA: 2013. Adenoviridae; pp. 1704–1731.
-
- Doucas V, et al. Adenovirus replication is coupled with the dynamic properties of the PML nuclear structure. Genes Dev. 1996;10(2):196–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

