Enantiomer excesses of rare and common sugar derivatives in carbonaceous meteorites
- PMID: 27247410
- PMCID: PMC4914185
- DOI: 10.1073/pnas.1603030113
Enantiomer excesses of rare and common sugar derivatives in carbonaceous meteorites
Abstract
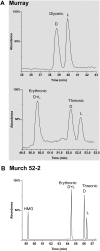
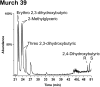
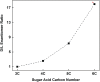
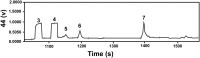
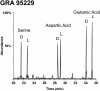
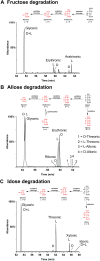
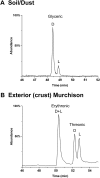
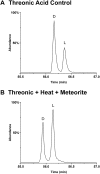
Biological polymers such as nucleic acids and proteins are constructed of only one-the d or l-of the two possible nonsuperimposable mirror images (enantiomers) of selected organic compounds. However, before the advent of life, it is generally assumed that chemical reactions produced 50:50 (racemic) mixtures of enantiomers, as evidenced by common abiotic laboratory syntheses. Carbonaceous meteorites contain clues to prebiotic chemistry because they preserve a record of some of the Solar System's earliest (∼4.5 Gy) chemical and physical processes. In multiple carbonaceous meteorites, we show that both rare and common sugar monoacids (aldonic acids) contain significant excesses of the d enantiomer, whereas other (comparable) sugar acids and sugar alcohols are racemic. Although the proposed origins of such excesses are still tentative, the findings imply that meteoritic compounds and/or the processes that operated on meteoritic precursors may have played an ancient role in the enantiomer composition of life's carbohydrate-related biopolymers.
Keywords: aldonic acids; carbonaceous meteorites; enantiomer excesses; polyols; sugar acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Cody G, Alexander CMO’D. NMR studies of chemical structural variation of insoluble organic matter from different carbonaceous chondrite groups. Geochim Cosmochim Acta. 2005;69(4):1085–1097.
-
- Alexander CMO’D, et al. Deuterium enrichments in chondritic macromolecular material-Implications for the origin and evolution of organics, water and asteroids. Geochim Cosmochim Acta. 2010;74(15):4417–4437.
-
- Pizzarello S, Cooper GW, Flynn GJ. The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In: Lauretta D, Leshin LA, McSween HY Jr, editors. Meteorites and the Early Solar System II. Univ Arizona Press; Tucson, AZ: 2006. pp. 625–651.
-
- Elsila JE, Charnley SB, Burton AS, Glavin DP, Dworkin JP. Compound specific carbon, nitrogen, and hydrogen isotopic ratios for amino acids in CM and CR chondrites and their use in evaluating potential formation pathways. Meteorit Planet Sci. 2012;47(9):1517–1536.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

