Adaptation of a plant pathogen to partial host resistance: selection for greater aggressiveness in grapevine downy mildew
- PMID: 27247621
- PMCID: PMC4869412
- DOI: 10.1111/eva.12368
Adaptation of a plant pathogen to partial host resistance: selection for greater aggressiveness in grapevine downy mildew
Abstract
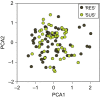
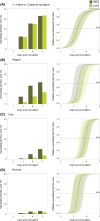
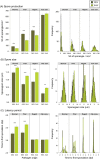
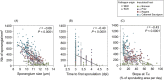
An understanding of the evolution of pathogen quantitative traits in response to host selective pressures is essential for the development of durable management strategies for resistant crops. However, we still lack experimental data on the effects of partial host resistance on multiple phenotypic traits (aggressiveness) and evolutionary strategies in pathogens. We performed a cross-inoculation experiment with four grapevine hosts and 103 isolates of grapevine downy mildew (Plasmopara viticola) sampled from susceptible and partially resistant grapevine varieties. We analysed the neutral and adaptive genetic differentiation of five quantitative traits relating to pathogen transmission. Isolates from resistant hosts were more aggressive than isolates from susceptible hosts, as they had a shorter latency period and higher levels of spore production. This pattern of adaptation contrasted with the lack of neutral genetic differentiation, providing evidence for directional selection. No specificity for a particular host variety was detected. Adapted isolates had traits that were advantageous on all resistant varieties. There was no fitness cost associated with this genetic adaptation, but several trade-offs between pathogen traits were observed. These results should improve the accuracy of prediction of fitness trajectories for this biotrophic pathogen, an essential element for the modelling of durable deployment strategies for resistant varieties.
Keywords: Vitis vinifera; erosion; evolvability; fitness cost; host specificity; obligate plant pathogen; quantitative resistance; virulence.
Figures
Similar articles
-
Rapid and multiregional adaptation to host partial resistance in a plant pathogenic oomycete: evidence from European populations of Plasmopara viticola, the causal agent of grapevine downy mildew.Infect Genet Evol. 2014 Oct;27:500-8. doi: 10.1016/j.meegid.2013.10.017. Epub 2013 Oct 30. Infect Genet Evol. 2014. PMID: 24184095
-
Breakdown of resistance to grapevine downy mildew upon limited deployment of a resistant variety.BMC Plant Biol. 2010 Jul 15;10:147. doi: 10.1186/1471-2229-10-147. BMC Plant Biol. 2010. PMID: 20633270 Free PMC article.
-
Transcriptional regulation of stilbene synthases in grapevine germplasm differentially susceptible to downy mildew.BMC Plant Biol. 2019 Sep 14;19(1):404. doi: 10.1186/s12870-019-2014-5. BMC Plant Biol. 2019. PMID: 31521112 Free PMC article.
-
Soft selective sweeps in fungicide resistance evolution: recurrent mutations without fitness costs in grapevine downy mildew.Mol Ecol. 2017 Apr;26(7):1936-1951. doi: 10.1111/mec.14006. Epub 2017 Jan 27. Mol Ecol. 2017. PMID: 28063192
-
Virulence Adaptation by Rice Planthoppers and Leafhoppers to Resistance Genes and Loci: A Review.Insects. 2024 Aug 29;15(9):652. doi: 10.3390/insects15090652. Insects. 2024. PMID: 39336620 Free PMC article. Review.
Cited by
-
Components of partial resistance to Plasmopara viticola enable complete phenotypic characterization of grapevine varieties.Sci Rep. 2020 Jan 17;10(1):585. doi: 10.1038/s41598-020-57482-0. Sci Rep. 2020. PMID: 31953499 Free PMC article.
-
Epidemiological trade-off between intra- and interannual scales in the evolution of aggressiveness in a local plant pathogen population.Evol Appl. 2018 Jan 4;11(5):768-780. doi: 10.1111/eva.12588. eCollection 2018 Jun. Evol Appl. 2018. PMID: 29875818 Free PMC article.
-
Pattern of local adaptation to quantitative host resistance in a major pathogen of a perennial crop.Evol Appl. 2019 Dec 31;13(4):824-836. doi: 10.1111/eva.12904. eCollection 2020 Apr. Evol Appl. 2019. PMID: 32211070 Free PMC article.
-
Modelling quantitative fungicide resistance and breakdown of resistant cultivars: Designing integrated disease management strategies for Septoria of winter wheat.PLoS Comput Biol. 2023 Mar 28;19(3):e1010969. doi: 10.1371/journal.pcbi.1010969. eCollection 2023 Mar. PLoS Comput Biol. 2023. PMID: 36976791 Free PMC article.
-
Tackling microbial threats in agriculture with integrative imaging and computational approaches.Comput Struct Biotechnol J. 2020 Dec 29;19:372-383. doi: 10.1016/j.csbj.2020.12.018. eCollection 2021. Comput Struct Biotechnol J. 2020. PMID: 33489007 Free PMC article. Review.
References
-
- Ahmed, S. , De Labrouhe D. T., and Delmotte F. 2012. Emerging virulence arising from hybridisation facilitated by multiple introductions of the sunflower downy mildew pathogen Plasmopara halstedii . Fungal Genetics and Biology 49:847–855. - PubMed
-
- Anderson, R. , and May R. 1982. Coevolution of hosts and parasites. Parasitology 85:411–426. - PubMed
-
- Andrivon, D. , Pilet F., Montarry J., Hafidi M., Corbière R., Achbani E. H., Pellé R. et al. 2007. Adaptation of Phytophthora infestans to partial resistance in potato: evidence from French and Moroccan populations. Phytopathology 97:338–343. - PubMed
-
- Antonovics, J. , and Alexander H. M. 1989. The concept of fitness in plant‐fungal pathogen systems In Fry W. E., and Leonard K. J., eds. Plant Disease Epidemiology, pp. 185–214. Mc Graw‐Hill, New‐York, NY.
-
- Aubertot, J. N. , West J. S., Bousset‐Vaslin L., Salam M. U., Barbetti M. J., and Diggle A. J. 2006. Improved resistance management for durable disease control: a case study of phoma stem canker of oilseed rape (Brassica napus). European Journal of Plant Pathology 114:91–106.
LinkOut - more resources
Full Text Sources
Other Literature Sources

