The protective effect of hydromorphone to ischemia in rat glial cells
- PMID: 27247906
- PMCID: PMC4864736
- DOI: 10.1186/s40064-016-2281-7
The protective effect of hydromorphone to ischemia in rat glial cells
Abstract
Background: Ischemic insults during operation can cause ischemic-reperfusion injuries in brain as well as memory impairments. Total intravenous anesthesia (TIVA) is the preferred anesthetic method in brain surgery, as it utilizes motor evoked potential monitoring. And the use of opioids is common in TIVA. However there are few studies about ischemic protective effect of opioids to glial cells.
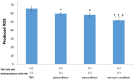
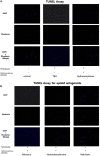
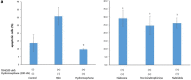
Methods: We used mixed cultures of rat glial cells, which were harvested from the brain of 1-day old rat. We divided the experimental groups according to their hydromorphone conditioning period: (a) pre-culture, (b) per-culture, or (c) pre- and per-culture. We measured the levels of the reactive oxygen species (ROS) induced by tert-butyl hydroperoxide (TBH) using flow cytometry. The ROS levels in the glial cells were also measured after the administration of 100 nM hydromorphone and selective opioid receptor antagonists.
Results: The ROS levels were reduced in the hydromorphone-treated group, as compared to the control group (only TBH treated). There were no differences between pre-conditioned and per-conditioned groups. However, the ROS levels were more reduced in pre- and per-conditioned group compared to pre-conditioned or per-conditioned only groups. Furthermore, selective antagonists for the delta, kappa, or mu opioid receptor partially negated the hydromorphone effect.
Conclusion: This study demonstrated that hydromorphone can have additional protective effects on oxidative stress when pre- and per-conditioning is combined. Furthermore we proved that μ, δ, κ opioid receptors participate in protective mechanism of hydromorphone to glial cells.
Keywords: Hydromorphone; Neuroglia; Reactive oxygen species.
Figures



References
-
- Askari N, Mahboudi F, Haeri-Rohani A, Kazemi B, Sarrami R, Edalat R, Ahmadiani A. Effects of single administration of morphine on G-protein mRNA level in the presence and absence of inflammation in the rat spinal cord. Scand J Immunol. 2008;67(1):47–52. doi: 10.1111/j.1365-3083.2007.02043.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

