Sideroblastic anemia: functional study of two novel missense mutations in ALAS2
- PMID: 27247955
- PMCID: PMC4867561
- DOI: 10.1002/mgg3.202
Sideroblastic anemia: functional study of two novel missense mutations in ALAS2
Abstract
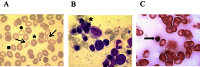
Background: X-linked sideroblastic anemia (XLSA) is a disorder characterized by decreased heme synthesis and mitochondrial iron overload with ringed sideroblasts in bone marrow. XLSA is caused by mutations in the erythroid-specific gene coding 5-aminolevulinate synthase (ALAS2). Anemia in XLSA is extremely variable, characteristically microcytic and hypochromic with poikilocytosis, and the red blood cell distribution width is increased and prominent dimorphism of the red cell population. Anemia in XLSA patients responds variably to supplementation with pyridoxine.
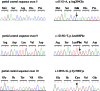
Methods and results: We report four patients with XLSA and three mutations in ALAS2: c.611G>A (p.Arg204Gln), c.1218G>T (p.Leu406Phe) and c.1499A>G (p.Tyr500Cys). The in silico predictions of three ALAS2 mutations and the functional consequences of two ALAS2 mutations were assessed. We performed in silico analysis of these mutations using ten different softwares, and all of them predicted that the p.Tyr500Cys mutation was deleterious. The in vitro prokaryotic expression showed that the p.Leu406Phe and p.Tyr500Cys mutations reduced the ALAS2 specific activity (SA) to 14% and 7% of the control value, respectively.
Conclusion: In view of the results obtained in this study, a clear relationship between genotype and phenotype cannot be established; clinical variability or severity of anemia may be influenced by allelic variants in other genes or transcription factors and environmental conditions.
Keywords: ALAS2; in silico analysis; prokaryotic expression; sideroblastic anemia.
Figures
References
-
- Aivado, M. , Gattermann N., Rong A., Giagounidis A. A., Prall W. C., Czibere A., et al. 2006. X‐linked sideroblastic anemia associated with a novel ALAS2 mutation and unfortunate skewed X‐chromosome inactivation patterns. Blood Cells Mol. Dis. 37:40–45. - PubMed
-
- Barton, J. C. , Lee P. L., Bertoli L. F., and Beutler E.. 2005. Iron overload in an African American woman with SS hemoglobinopathy and a promoter mutation in the X‐linked erythroid‐specific 5‐aminolevulinate synthase (ALAS2) gene. Blood Cells Mol. Dis. 34:226–228. - PubMed
-
- Bekri, S. , May A., Cotter P. D., Al‐Sabah A. I., Guo X., Masters G. S., et al. 2003. A promoter mutation in the erythroid‐specific 5‐aminolevulinate synthase (ALAS2) gene causes X‐linked sideroblastic anemia. Blood 102:698–704. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

