Carrier screening by next-generation sequencing: health benefits and cost effectiveness
- PMID: 27247957
- PMCID: PMC4867563
- DOI: 10.1002/mgg3.204
Carrier screening by next-generation sequencing: health benefits and cost effectiveness
Abstract
Background: Compared with conventional genotyping, which typically tests for a limited number of mutations, next-generation DNA sequencing (NGS) provides increased accuracy for carrier screening. The objective of this study was to evaluate the cost effectiveness of carrier screening using NGS versus genotyping for 14 of the recessive disorders for which medical society guidelines recommend screening.
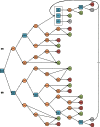
Methods: Data from published literature, population surveys, and expert opinion were used to develop a decision tree model capturing decisions and outcomes related to carrier screening and reproductive health.
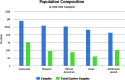
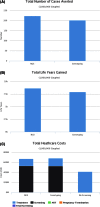
Results: Modeling a population of 1,000,000 couples that was representative of the United States population and that contained 83,421 carriers of pathogenic mutations, carrier screening using NGS averted 21 additional affected births as compared with genotyping, and reduced costs by approximately $13 million. As compared with no screening, NGS carrier screening averted 223 additional affected births. The results are sensitive to assumptions regarding mutation detection rates and carrier frequencies in multiethnic populations.
Conclusion: This study demonstrated that NGS-based carrier screening offers the greater benefit in clinical outcomes and lower total healthcare cost as compared with genotyping.
Keywords: Carrier screening; cost effectiveness; genotyping; next‐generation sequencing.
Figures
References
-
- American Congress of Obstetricians and Gynecologists . 2009. ACOG Committee Opinion No. 442: preconception and prenatal carrier screening for genetic diseases in individuals of Eastern European Jewish descent. Obstet. Gynecol. 114:950–953. - PubMed
-
- American Congress of Obstetricians and Gynecologists . 2011. ACOG Committee Opinion No. 486: update on carrier screening for cystic fibrosis. Obstet. Gynecol. 117:1028–1031. - PubMed
-
- Amos, J. , Feldman G. L., Grody W. W., Monaghan K., Palomaki G. E., and Prior T.. 2006. American College of Medical Genetics: technical standards and guidelines for CFTR mutation testing: 2006 Edition [Online]. American College of Medical Genetics, Bethesda, MD: Available at https://www.acmg.net/Pages/ACMG_Activities/stds-2002/cf.htm (accessed 20 June 2014).
-
- Arias, E. 2014. United States life tables, 2009. National Center for Health Statistics, Hyattsville, MD: Available at http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_07.pdf. Natl. Vital Stat. Rep. 62:1–63. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

