Targeting innate immunity for neurodegenerative disorders of the central nervous system
- PMID: 27248001
- PMCID: PMC5433264
- DOI: 10.1111/jnc.13667
Targeting innate immunity for neurodegenerative disorders of the central nervous system
Erratum in
-
Erratum.J Neurochem. 2017 Apr;141(1):151. doi: 10.1111/jnc.13984. Epub 2017 Feb 24. J Neurochem. 2017. PMID: 28332228 No abstract available.
Abstract
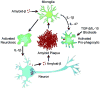
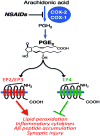
Neuroinflammation is critically involved in numerous neurodegenerative diseases, and key signaling steps of innate immune activation hence represent promising therapeutic targets. This mini review series originated from the 4th Venusberg Meeting on Neuroinflammation held in Bonn, Germany, 7-9th May 2015, presenting updates on innate immunity in acute brain injury and chronic neurodegenerative disorders, such as traumatic brain injury and Alzheimer disease, on the role of astrocytes and microglia, as well as technical developments that may help elucidate neuroinflammatory mechanisms and establish clinical relevance. In this meeting report, a brief overview of physiological and pathological microglia morphology is followed by a synopsis on PGE2 receptors, insights into the role of arginine metabolism and further relevant aspects of neuroinflammation in various clinical settings, and concluded by a presentation of technical challenges and solutions when working with microglia and astrocyte cultures. Microglial ontogeny and induced pluripotent stem cell-derived microglia, advances of TREM2 signaling, and the cytokine paradox in Alzheimer's disease are further contributions to this article. Neuroinflammation is critically involved in numerous neurodegenerative diseases, and key signaling steps of innate immune activation hence represent promising therapeutic targets. This mini review series originated from the 4th Venusberg Meeting on Neuroinflammation held in Bonn, Germany, 7-9th May 2015, presenting updates on innate immunity in acute brain injury and chronic neurodegenerative disorders, such as traumatic brain injury and Alzheimer's disease, on the role of astrocytes and microglia, as well as technical developments that may help elucidate neuroinflammatory mechanisms and establish clinical relevance. In this meeting report, a brief overview on physiological and pathological microglia morphology is followed by a synopsis on PGE2 receptors, insights into the role of arginine metabolism and further relevant aspects of neuroinflammation in various clinical settings, and concluded by a presentation of technical challenges and solutions when working with microglia cultures. Microglial ontogeny and induced pluripotent stem cell-derived microglia, advances of TREM2 signaling, and the cytokine paradox in Alzheimer's disease are further contributions to this article.
Keywords: Alzheimer disease; Venusberg Neuroinflammation Meeting Bonn 2015; blood-brain barrier; innate immunity; macrophage; non-steroidal anti-inflammatory drugs (NSAIDs).
© 2016 International Society for Neurochemistry.
Conflict of interest statement
The authors have no conflict of interests to declare. All experiments were conducted with the ARRIVE guidelines.
Figures


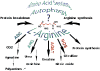


References
-
- Abutbul S, Shapiro J, et al. TGF-beta signaling through SMAD2/3 induces the quiescent microglial phenotype within the CNS environment. Glia. 2012;60(7):1160–1171. - PubMed
-
- Aisen PS, Davis KL, et al. A randomized controlled trial of prednisone in Alzheimer’s disease. Alzheimer’s Disease Cooperative Study. Neurology. 2000;54(3):588–593. - PubMed
Publication types
MeSH terms
Grants and funding
- R21 NS048522/NS/NINDS NIH HHS/United States
- R01 NS074804/NS/NINDS NIH HHS/United States
- R01 NS076794/NS/NINDS NIH HHS/United States
- K99 AG044445/AG/NIA NIH HHS/United States
- R01 AG030149/AG/NIA NIH HHS/United States
- P50 AG047366/AG/NIA NIH HHS/United States
- RF1 AG051485/AG/NIA NIH HHS/United States
- R01 NS093920/NS/NINDS NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- R01 AG030209/AG/NIA NIH HHS/United States
- U01 AG050636/AG/NIA NIH HHS/United States
- R01 AG023012/AG/NIA NIH HHS/United States
- R21 AG033914/AG/NIA NIH HHS/United States
- R01 AG043522/AG/NIA NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

