Angiotensin-Converting Enzyme Inhibition as an Adjunct to Pulmonary Rehabilitation in Chronic Obstructive Pulmonary Disease
- PMID: 27248440
- PMCID: PMC5148142
- DOI: 10.1164/rccm.201601-0094OC
Angiotensin-Converting Enzyme Inhibition as an Adjunct to Pulmonary Rehabilitation in Chronic Obstructive Pulmonary Disease
Abstract
Rationale: Epidemiological studies in older individuals have found an association between the use of angiotensin-converting enzyme (ACE) inhibition (ACE-I) therapy and preserved locomotor muscle mass, strength, and walking speed. ACE-I therapy might therefore have a role in the context of pulmonary rehabilitation (PR).
Objectives: To investigate the hypothesis that enalapril, an ACE inhibitor, would augment the improvement in exercise capacity seen during PR.
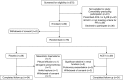
Methods: We performed a double-blind, placebo-controlled, parallel-group randomized controlled trial. Patients with chronic obstructive pulmonary disease, who had at least moderate airflow obstruction and were taking part in PR, were randomized to either 10 weeks of therapy with an ACE inhibitor (10 mg enalapril) or placebo.
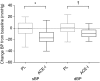
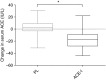
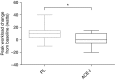
Measurements and main results: The primary outcome measurement was the change in peak power (assessed using cycle ergometry) from baseline. Eighty patients were enrolled, 78 were randomized (age 67 ± 8 years; FEV1 48 ± 21% predicted), and 65 completed the trial (34 on placebo, 31 on the ACE inhibitor). The ACE inhibitor-treated group demonstrated a significant reduction in systolic blood pressure (Δ, -16 mm Hg; 95% confidence interval [CI], -22 to -11) and serum ACE activity (Δ, -18 IU/L; 95% CI, -23 to -12) versus placebo (between-group differences, P < 0.0001). Peak power increased significantly more in the placebo group (placebo Δ, +9 W; 95% CI, 5 to 13 vs. ACE-I Δ, +1 W; 95% CI, -2 to 4; between-group difference, 8 W; 95% CI, 3 to 13; P = 0.001). There was no significant between-group difference in quadriceps strength or health-related quality of life.
Conclusions: Use of the ACE inhibitor enalapril, together with a program of PR, in patients without an established indication for ACE-I, reduced the peak work rate response to exercise training in patients with chronic obstructive pulmonary disease.
Keywords: COPD; exercise; rehabilitation; renin–angiotensin system.
Figures
Comment in
-
Surprising Results from an Angiotensin-Converting Enzyme Inhibitor Trial in Patients with Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med. 2016 Dec 1;194(11):1307-1308. doi: 10.1164/rccm.201606-1205ED. Am J Respir Crit Care Med. 2016. PMID: 27905853 No abstract available.
References
-
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153:976–980. - PubMed
-
- Shrikrishna D, Patel M, Tanner RJ, Seymour JM, Connolly BA, Puthucheary ZA, Walsh SL, Bloch SA, Sidhu PS, Hart N, et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J. 2012;40:1115–1122. - PubMed
-
- Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, Carone M, Celli B, Engelen M, Fahy B, et al. ATS/ERS Pulmonary Rehabilitation Writing Committee. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–1413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

