A GCN2-Like eIF2α Kinase (LdeK1) of Leishmania donovani and Its Possible Role in Stress Response
- PMID: 27248816
- PMCID: PMC4889150
- DOI: 10.1371/journal.pone.0156032
A GCN2-Like eIF2α Kinase (LdeK1) of Leishmania donovani and Its Possible Role in Stress Response
Abstract
Translation regulation in Leishmania parasites assumes significance particularly because they encounter myriad of stresses during their life cycle. The eukaryotic initiation factor 2α (eIF2α) kinases, the well-known regulators of translation initiation in higher eukaryotes have now been found to control various processes in these protozoan parasites as well. Here, we report on cloning and characterization of a GCN2-like eIF2α kinase from L. donovani and also on its modulation during nutrient starvation. We cloned a GCN2-like kinase from L. donovani, which we named as LdeK1 and validated it to be a functional eIF2α kinase by in vitro kinase assay. LdeK1 was found to be localized in the cytoplasm of the promastigotes with a five-fold higher expression in this stage of the parasite as compared to the axenic amastigotes. Phosphorylation of eIF2α and a G1-arrest was observed in response to nutrient starvation in the wild-type parasites. In contrast, phosphorylation was significantly impaired in a dominant-negative mutant of LdeK1 during this stress with a subsequent failure to bring about a G1-arrest during cell cycle. Thus, LdeK1 is a functional GCN2-like kinase of L. donovani which responds to nutrient starvation by phosphorylating its substrate, eIF2α and a G1-arrest in the cell cycle. Nutrient starvation is encountered by the parasites inside the vector which triggers metacyclogenesis. We therefore propose that global translational regulation by activation of LdeK1 followed by eIF2α phosphorylation and G1-arrest during nutrient starvation in the gut of sandfly vector could be one of the mechanisms to retool the cellular machinery required for metacyclogenesis of Leishmania promastigotes.
Conflict of interest statement
Figures





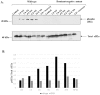
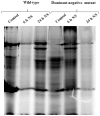
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

