Unraveling the mechanisms of chromatin fibril packaging
- PMID: 27249516
- PMCID: PMC4991243
- DOI: 10.1080/19491034.2016.1190896
Unraveling the mechanisms of chromatin fibril packaging
Abstract
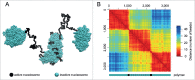
Recent data indicate that eukaryotic chromosomes are organized into Topologically Associating Domains (TADs); however, the mechanisms underlying TAD formation remain obscure. Based on the results of Hi-C analysis performed on 4 Drosophila melanogaster cell lines, we have proposed that specific properties of nucleosomes in active and repressed chromatin play a key role in the formation of TADs. Our computer simulations showed that the ability of "inactive" nucleosomes to stick to each other and the lack of such ability in "active" nucleosomes is sufficient for spatial segregation of these types of chromatin, which is revealed in the Hi-C analysis as TAD/inter-TAD partitioning. However, some Drosophila and mammalian TADs contain both active and inactive chromatin, a fact that does not fit this model. Herein, we present additional arguments for the model by postulating that transcriptionally active chromatin is extruded on the surface of a TAD, and discuss the possible impact of this organization on the enhancer-promoter communication and on the segregation of TADs.
Keywords: chromatin spatial structure; nucleosome; polymer simulations; topologically associating domains; transcription.
Figures

Comment on
- Extra view to:Ulianov SV, Khrameeva EE, Gavrilov AA, Flyamer IM, Kos P, Mikhaleva EA, Penin AA, Logacheva MD, Imakaev MV, Chertovich A, et al. . Active chromatin and transcription play a key role in chromosome partitioning into topologically associating domains. Genome Res 2016; 26:70-84. PMID: ; http://dx.doi.org/10.1101/gr.196006.115
References
-
- Maeshima K, Ide S, Hibino K, Sasai M. Liquid-like behavior of chromatin. Curr Opin Genet Dev 2016; 37:36-45; PMID:26826680; http://dx.doi.org/10.1016/j.gde.2015.11.006 - DOI - PubMed
-
- Razin SV, Gavrilov AA. Chromatin without the 30-nm fiber: constrained disorder instead of hierarchical folding. Epigenetics 2014; 9:653-7; PMID:24561903; http://dx.doi.org/10.4161/epi.28297 - DOI - PMC - PubMed
-
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, et al.. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 2009; 326:289-93; PMID:19815776; http://dx.doi.org/10.1126/science.1181369 - DOI - PMC - PubMed
-
- Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 2012; 485:376-80; PMID:22495300; http://dx.doi.org/10.1038/nature11082 - DOI - PMC - PubMed
-
- Nora EP, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, Servant N, Piolot T, van Berkum NL, Meisig J, Sedat J, et al.. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 2012; 485:381-5; PMID:22495304; http://dx.doi.org/10.1038/nature11049 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
