Active Surveillance of Prostate Cancer: Use, Outcomes, Imaging, and Diagnostic Tools
- PMID: 27249729
- PMCID: PMC4917301
- DOI: 10.1200/EDBK_159244
Active Surveillance of Prostate Cancer: Use, Outcomes, Imaging, and Diagnostic Tools
Abstract
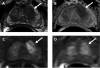
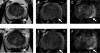
Active surveillance (AS) has emerged as a standard management option for men with very low-risk and low-risk prostate cancer, and contemporary data indicate that use of AS is increasing in the United States and abroad. In the favorable-risk population, reports from multiple prospective cohorts indicate a less than 1% likelihood of metastatic disease and prostate cancer-specific mortality over intermediate-term follow-up (median 5-6 years). Higher-risk men participating in AS appear to be at increased risk of adverse outcomes, but these populations have not been adequately studied to this point. Although monitoring on AS largely relies on serial prostate biopsy, a procedure associated with considerable morbidity, there is a need for improved diagnostic tools for patient selection and monitoring. Revisions from the 2014 International Society of Urologic Pathology consensus conference have yielded a more intuitive reporting system and detailed reporting of low-intermediate grade tumors, which should facilitate the practice of AS. Meanwhile, emerging modalities such as multiparametric magnetic resonance imaging and tissue-based molecular testing have shown prognostic value in some populations. At this time, however, these instruments have not been sufficiently studied to consider their routine, standardized use in the AS setting. Future studies should seek to identify those platforms most informative in the AS population and propose a strategy by which promising diagnostic tools can be safely and efficiently incorporated into clinical practice.
Figures

References
-
- Choo R, Klotz L, Danjoux C, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J. Urol. 2002;167:1664–1669. - PubMed
-
- Carter HB, Walsh PC, Landis P, et al. Expectant management of nonpalpable prostate cancer with curative intent: preliminary results. J Urol. 2002;167:1231–1234. - PubMed
-
- Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur. Urol. 2014;65:124–37. - PubMed
-
- Mohler J, Armstrong A, Bahnson R, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline): Prostate Cancer. Version 1 2015.
-
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-Term Follow-Up of a Large Active Surveillance Cohort of Patients With Prostate Cancer. J. Clin. Oncol. 2015;33:272–277. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

