The CebE/MsiK Transporter is a Doorway to the Cello-oligosaccharide-mediated Induction of Streptomyces scabies Pathogenicity
- PMID: 27250236
- PMCID: PMC4890002
- DOI: 10.1038/srep27144
The CebE/MsiK Transporter is a Doorway to the Cello-oligosaccharide-mediated Induction of Streptomyces scabies Pathogenicity
Abstract
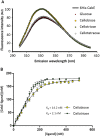
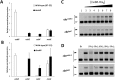
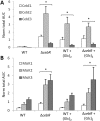
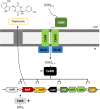
Streptomyces scabies is an economically important plant pathogen well-known for damaging root and tuber crops by causing scab lesions. Thaxtomin A is the main causative agent responsible for the pathogenicity of S. scabies and cello-oligosaccharides are environmental triggers that induce the production of this phytotoxin. How cello-oligosaccharides are sensed or transported in order to induce the virulent behavior of S. scabies? Here we report that the cellobiose and cellotriose binding protein CebE, and MsiK, the ATPase providing energy for carbohydrates transport, are the protagonists of the cello-oligosaccharide mediated induction of thaxtomin production in S. scabies. Our work provides the first example where the transport and not the sensing of major constituents of the plant host is the central mechanism associated with virulence of the pathogen. Our results allow to draw a complete pathway from signal transport to phytotoxin production where each step of the cascade is controlled by CebR, the cellulose utilization regulator. We propose the high affinity of CebE to cellotriose as possible adaptation of S. scabies to colonize expanding plant tissue. Our work further highlights how genes associated with primary metabolism in nonpathogenic Streptomyces species have been recruited as basic elements of virulence in plant pathogenic species.
Figures



References
-
- Hopwood D. A. Streptomyces in Nature and Medicine: The Antibiotic Makers. (Oxford University Press, 2007).
-
- Hodgson D. A. Primary metabolism and its control in streptomycetes: a most unusual group of bacteria. Adv. Microb. Physiol. 42, 47–238 (2000). - PubMed
-
- Francis I., Holsters M. & Vereecke D. The Gram-positive side of plant-microbe interactions. Environ. Microbiol. 12, 1–12 (2010). - PubMed
-
- Kinkel L. L., Schlatter D. C., Bakker M. G. & Arenz B. E. Streptomyces competition and co-evolution in relation to plant disease suppression. Res. Microbiol. 163, 490–499 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

