Genetically Engineered Phages: a Review of Advances over the Last Decade
- PMID: 27250768
- PMCID: PMC4981678
- DOI: 10.1128/MMBR.00069-15
Genetically Engineered Phages: a Review of Advances over the Last Decade
Abstract
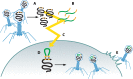
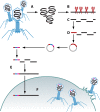
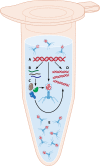
Soon after their discovery in the early 20th century, bacteriophages were recognized to have great potential as antimicrobial agents, a potential that has yet to be fully realized. The nascent field of phage therapy was adversely affected by inadequately controlled trials and the discovery of antibiotics. Although the study of phages as anti-infective agents slowed, phages played an important role in the development of molecular biology. In recent years, the increase in multidrug-resistant bacteria has renewed interest in the use of phages as antimicrobial agents. With the wide array of possibilities offered by genetic engineering, these bacterial viruses are being modified to precisely control and detect bacteria and to serve as new sources of antibacterials. In applications that go beyond their antimicrobial activity, phages are also being developed as vehicles for drug delivery and vaccines, as well as for the assembly of new materials. This review highlights advances in techniques used to engineer phages for all of these purposes and discusses existing challenges and opportunities for future work.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- d'Hérelle F. 1917. Sur un microbe invisible antagoniste des bacilles dysentériques. C R Hebd Seances Acad Sci Ser D 165:373–375.
-
- Bruynoghe R, Maisin J. 1921. Essais de thérapeutique au moyen du bacteriophage. C R Soc Biol 85:1120–1121.
-
- d'Hérelle F. 1921. Le bactériophage: son rôle dans l'immunité. Masson et Cie, Paris, France.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

