Tetrahymena Poc1 ensures proper intertriplet microtubule linkages to maintain basal body integrity
- PMID: 27251062
- PMCID: PMC4966981
- DOI: 10.1091/mbc.E16-03-0165
Tetrahymena Poc1 ensures proper intertriplet microtubule linkages to maintain basal body integrity
Abstract
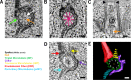
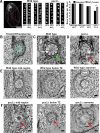
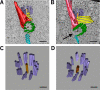
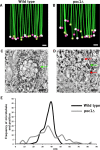
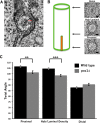
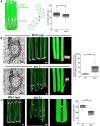
Basal bodies comprise nine symmetric triplet microtubules that anchor forces produced by the asymmetric beat pattern of motile cilia. The ciliopathy protein Poc1 stabilizes basal bodies through an unknown mechanism. In poc1∆ cells, electron tomography reveals subtle defects in the organization of intertriplet linkers (A-C linkers) that connect adjacent triplet microtubules. Complete triplet microtubules are lost preferentially near the posterior face of the basal body. Basal bodies that are missing triplets likely remain competent to assemble new basal bodies with nine triplet microtubules, suggesting that the mother basal body microtubule structure does not template the daughter. Our data indicate that Poc1 stabilizes basal body triplet microtubules through linkers between neighboring triplets. Without this stabilization, specific triplet microtubules within the basal body are more susceptible to loss, probably due to force distribution within the basal body during ciliary beating. This work provides insights into how the ciliopathy protein Poc1 maintains basal body integrity.
© 2016 Meehl et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures
References
-
- Abal M, Keryer G, Bornens M. Centrioles resist forces applied on centrosomes during G2/M transition. Biol Cell. 2005;97:425–434. - PubMed
-
- Albrecht-Buehler G. The iris diaphragm model of centriole and basal body formation. Cell Motil Cytoskeleton. 1990;17:197–213. - PubMed
-
- Alvey PL. Do adult centrioles contain cartwheels and lie at right angles to each other. Cell Biol Int Rep. 1986;10:589–598. - PubMed
-
- Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

