Antipsychotics for fibromyalgia in adults
- PMID: 27251337
- PMCID: PMC6457603
- DOI: 10.1002/14651858.CD011804.pub2
Antipsychotics for fibromyalgia in adults
Abstract
Background: This review is one of a series on drugs used to treat fibromyalgia. Fibromyalgia is a clinically well-defined chronic condition of unknown aetiology characterised by chronic widespread pain that often co-exists with sleep problems and fatigue. It affects approximately 2% of the general population. Up to 70% of patients with fibromyalgia meet the criteria for a depressive or anxiety disorder. People often report high disability levels and poor health-related quality of life. Drug therapy focuses on reducing key symptoms and disability, and improving health-related quality of life. Antipsychotics might reduce fibromyalgia and associated mental health symptoms.
Objectives: To assess the efficacy, tolerability and safety of antipsychotics in fibromyalgia in adults.
Search methods: We searched CENTRAL (2016, Issue 4), MEDLINE and EMBASE to 20 May 2016, together with reference lists of retrieved papers and reviews and two clinical trial registries. We also contacted trial authors.
Selection criteria: We selected controlled trials of at least four weeks duration of any formulation of antipsychotics used for the treatment of fibromyalgia in adults.
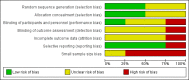
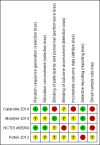
Data collection and analysis: We extracted the data from all included studies and two review authors independently assessed study risks of bias. We resolved discrepancies by discussion. We performed analysis using three tiers of evidence. We derived first tier evidence from data meeting current best standards and subject to minimal risk of bias (outcome equivalent to substantial pain intensity reduction, intention-to-treat analysis without imputation for drop-outs, at least 200 participants in the comparison, eight to 12 weeks duration, parallel design), second tier evidence from data that failed to meet one or more of these criteria and that we considered at some risk of bias but with adequate numbers in the comparison, and third tier evidence from data involving small numbers of participants that we considered very likely to be biased or used outcomes of limited clinical utility, or both. We rated the quality of evidence using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.
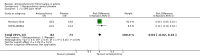
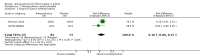
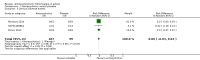
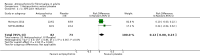
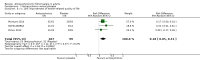
Main results: We included a total of four studies with 296 participants.Three studies with 206 participants compared quetiapine, an atypical (second-generation) antipsychotic, with placebo. One study used a cross-over design and two studies a parallel-group design. Study duration was eight or 12 weeks. Quetiapine was used in all studies with a bedtime dosage between 50 and 300 mg/day. All studies had one or more sources of potential major bias and we judged them to be at moderate risk of bias overall. The primary outcomes in this review were participant-reported pain relief of 50% or greater, Patient Global Impression of Change (PGIC) much or very much improved, withdrawal due to adverse events (tolerability) and serious adverse events (safety).Second tier evidence indicated that quetiapine was not statistically superior to placebo in the number of participants with a 50% or more pain reduction (very low quality evidence). No study reported data on PGIC. A greater proportion of participants on quetiapine reported a 30% or more pain reduction (risk difference (RD) 0.12, 95% confidence interval (CI) 0.00 to 0.23; number needed to treat for an additional benefit (NNTB) 8, 95% CI 5 to 100) (very low quality evidence). A greater proportion of participants on quetiapine reported a clinically relevant improvement of health-related quality of life compared to placebo ( RD 0.18, 95% CI 0.05 to 0.31; NNTB 5, 95% CI 3 to 20) (very low quality evidence). Quetiapine was statistically superior to placebo in reducing sleep problems (standardised mean difference (SMD) -0.67, 95% CI -1.10 to -0.23), depression (SMD -0.39, 95% CI -0.74 to -0.04) and anxiety (SMD -0.40, 95% CI -0.69 to -0.11) (very low quality evidence). Quetiapine was statistically superior to placebo in reducing the risk of withdrawing from the study due to a lack of efficacy (RD -0.14, 95% CI -0.23 to -0.05) (very low quality evidence). There was no statistically significant difference between quetiapine and placebo in the proportion of participants withdrawing due to adverse events (tolerability) (very low quality evidence), in the frequency of serious adverse events (safety) (very low quality evidence) and in the proportion of participants reporting dizziness and somnolence as an adverse event (very low quality evidence). In more participants in the quetiapine group a substantial weight gain was noted (RD 0.08, 95% CI 0.02 to 0.15; number needed to treat for an additional harm (NNTH) 12, 95% CI 6 to 50) (very low quality evidence). We downgraded the quality of evidence by three levels to a very low quality rating because of limitations of study design, indirectness (patients with major medical diseases and mental disorders were excluded) and imprecision (fewer than 400 patients were analysed).One parallel design study with 90 participants compared quetiapine (50 to 300 mg/day flexible at bedtime) to amitriptyline (10 to 75 mg/day flexible at bedtime). The study had three major risks of bias and we judged it to be at moderate risk of bias overall. We downgraded the quality of evidence by two levels to a low quality rating because of indirectness (patients with major medical diseases and mental disorders were excluded) and imprecision (fewer than 400 patients were analysed). Third tier evidence indicated no statistically significant differences between the two drugs. Both drugs did not statistically significantly differ in the reduction of average scores for pain, fatigue, sleep problems, depression, anxiety and for limitations of health-related quality of life and in the proportion of participants reporting dizziness, somnolence and weight gain as a side effect (low quality evidence). Compared to amitriptyline, more participants left the study due to adverse events (low quality evidence). No serious adverse events were reported (low quality evidence).We found no relevant study with other antipsychotics than quetiapine in fibromyalgia.
Authors' conclusions: Very low quality evidence suggests that quetiapine may be considered for a time-limited trial (4 to 12 weeks) to reduce pain, sleep problems, depression and anxiety in fibromyalgia patients with major depression. Potential side effects such as weight gain should be balanced against the potential benefits in shared decision making with the patient.
Conflict of interest statement
BW none known; BW is a specialist pain physician and manages patients with fibromyalgia.
PK: none known.
NÜ is a neurologist and pain physician who treats patients with fibromyalgia. She is member of the German guideline group on fibromyalgia. She received travel grants, research support and speaker honoraria from Genzyme (2014, 2015, 2016). She received a travel grant from Shire (2014, 2015). She received speaker honoraria from Baxalta (2014, 2015).
TP none known; TP is a specialist pain physician and manages patients with fibromyalgia.
WH is a specialist in general internal medicine, psychosomatic medicine and pain medicine, who treats patients with fibromyalgia. He is a member of the medical board of the German Fibromyalgia Association. He is the head of the steering committee of the German guideline on fibromyalgia and a member of the steering committee of the European League Against Rheumatism (EULAR) update recommendations on the management of fibromyalgia. He received speaking fees for one educational lecture each from MSD Sharpe & Dohme (2014) and Grünenthal (2015) on pain management.
Figures



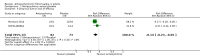

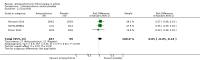
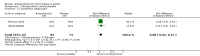
Update of
- doi: 10.1002/14651858.CD011804
Similar articles
-
Cannabinoids for fibromyalgia.Cochrane Database Syst Rev. 2016 Jul 18;7(7):CD011694. doi: 10.1002/14651858.CD011694.pub2. Cochrane Database Syst Rev. 2016. PMID: 27428009 Free PMC article.
-
Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia.Cochrane Database Syst Rev. 2018 Feb 28;2(2):CD010292. doi: 10.1002/14651858.CD010292.pub2. Cochrane Database Syst Rev. 2018. PMID: 29489029 Free PMC article.
-
Mirtazapine for fibromyalgia in adults.Cochrane Database Syst Rev. 2018 Aug 6;8(8):CD012708. doi: 10.1002/14651858.CD012708.pub2. Cochrane Database Syst Rev. 2018. PMID: 30080242 Free PMC article.
-
Oxycodone for neuropathic pain and fibromyalgia in adults.Cochrane Database Syst Rev. 2014 Jun 23;(6):CD010692. doi: 10.1002/14651858.CD010692.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2016 Jul 28;7:CD010692. doi: 10.1002/14651858.CD010692.pub3. PMID: 24956205 Updated.
-
Gabapentin for chronic neuropathic pain and fibromyalgia in adults.Cochrane Database Syst Rev. 2014 Apr 27;2014(4):CD007938. doi: 10.1002/14651858.CD007938.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Jun 09;6:CD007938. doi: 10.1002/14651858.CD007938.pub4. PMID: 24771480 Free PMC article. Updated.
Cited by
-
Efficacy of cupping therapy in patients with the fibromyalgia syndrome-a randomised placebo controlled trial.Sci Rep. 2016 Nov 17;6:37316. doi: 10.1038/srep37316. Sci Rep. 2016. PMID: 27853272 Free PMC article. Clinical Trial.
-
Comparative Analysis of Psychophysiological Responses in Fibromyalgia Patients: Evaluating Neuromodulation Alone, Neuromodulation Combined with Virtual Reality, and Exercise Interventions.Medicina (Kaunas). 2024 Feb 27;60(3):404. doi: 10.3390/medicina60030404. Medicina (Kaunas). 2024. PMID: 38541130 Free PMC article. Clinical Trial.
-
Beyond Amitriptyline: A Pediatric and Adolescent Oriented Narrative Review of the Analgesic Properties of Psychotropic Medications for the Treatment of Complex Pain and Headache Disorders.Children (Basel). 2020 Dec 2;7(12):268. doi: 10.3390/children7120268. Children (Basel). 2020. PMID: 33276542 Free PMC article. Review.
-
Facts and myths pertaining to fibromyalgia.Dialogues Clin Neurosci. 2018 Mar;20(1):53-62. doi: 10.31887/DCNS.2018.20.1/whauser. Dialogues Clin Neurosci. 2018. PMID: 29946212 Free PMC article. Review.
-
Pharmacological Treatment of Fibromyalgia Syndrome: A Practice-Based Review.Curr Pain Headache Rep. 2024 Dec;28(12):1349-1363. doi: 10.1007/s11916-024-01277-9. Epub 2024 Jul 23. Curr Pain Headache Rep. 2024. PMID: 39042299 Free PMC article. Review.
References
References to studies included in this review
Calandre 2014 {published data only}
-
- Calandre EP, Rico‐Villademoros F, Galán J, Molina‐Barea R, Vilchez JS, et al. Quetiapine extended‐release (Seroquel‐XR) versus amitriptyline monotherapy for treating patients with fibromyalgia: a 16‐week, randomized, flexible‐dose, open‐label trial. Psychopharmacology 2014;231:2525‐31. - PubMed
McIntyre 2014 {published data only}
-
- McIntyre A, Paisley D, Kouassi E, Gendron A. Quetiapine fumarate extended‐release for the treatment of major depression with comorbid fibromyalgia syndrome: a double‐blind, randomized, placebo‐controlled study. Arthritis and Rheumatism 2014;66:455‐61. - PubMed
NCT01458964 {published and unpublished data}
-
- NCT01458964. Quetiapine compared with placebo in the management of fibromyalgia. https://clinicaltrials.gov/ct2/show/NCT01458964?term=quetiapine+and+fibr... 8 July 2014; data provided on request.
Potvin 2012 {published data only}
-
- Potvin S, Morin M, Cloutier C, Gendron A, Bissonnette A, Marchand S. Add‐on treatment of quetiapine for fibromyalgia: a pilot, randomized, double‐blind, placebo‐controlled 12‐week trial. Journal of Clinical Psychopharmacology 2012;32:684‐7. - PubMed
References to studies excluded from this review
Moldofsky 1980 {published data only}
-
- Moldofsky H, Lue FA. The relationship of alpha and delta EEG frequencies to pain and mood in 'fibrositis' patients treated with chlorpromazine and L‐tryptophan. Electroencephalography Clinical Neurophysiology 1980;50:71‐80. - PubMed
Additional references
Ablin 2013
-
- Ablin J, Fitzcharles MA, Buskila D, Shir Y, Sommer C, et al. Treatment of fibromyalgia syndrome: recommendations of recent evidence‐based interdisciplinary guidelines with special emphasis on complementary and alternative therapies. Evidence Based Complementary Alternative Medicine 2013;2013:485272. - PMC - PubMed
Ammar 2015
-
- Ammar G, Naja WJ, Pelissolo A. Treatment‐resistant anxiety disorders: a literature review of drug therapy strategies. Encephale 2015;41(3):260‐5. - PubMed
Arnold 2013
Bennett 2009
-
- Bennett RM, Bushmakin AG, Cappelleri JC, Zlateva G, Sadosky AB. Minimal clinically important difference in the fibromyalgia impact questionnaire. Journal of Rheumatology 2009;36:1304‐11. - PubMed
Bradley 2009
Calandre 2012
-
- Calandre EP, Rico‐Villademoros F. The role of antipsychotics in the management of fibromyalgia. CNS Drugs 2012;26:135‐53. - PubMed
Choi 2010
Clauw 2014
-
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547‐55. - PubMed
Cochrane PaPaS Group 2011
-
- Cochrane Pain, Palliative & Supportive Care Review Group. PaPaS Author and Referee Guidance. Authoring or assessing a Cochrane Protocol, Review, or Review Update for the PaPaS Review Group. Document version 1. September 2011. http://papas.cochrane.org/papas‐documents (accessed 22 August 2015).
Cohen 1988
-
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates, 1998.
Dworkin 2008
Edwards 2013
Eich 2012
-
- Eich W, Häuser W, Arnold B, Bernardy K, Brückle W, Eidmann U, et al. Fibromyalgia syndrome. General principles and coordination of clinical care and patient education. Schmerz 2012;26:268‐75. - PubMed
Fayers 2014
Fitzcharles 2013
Forseth 1999
-
- Forseth KO, Husby G, Gran JT, Førre O. Prognostic factors for the development of fibromyalgia in women with self‐reported musculoskeletal pain. A prospective study. Journal of Rheumatology 1999;26:2458‐67. - PubMed
Furukawa 2005
-
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta‐analyses. International Clinical Psychopharmacology 2005;20:49‐52. - PubMed
Galek 2013
-
- Galek A, Erbslöh‐Möller B, Köllner V, Kühn‐Becker H, Langhorst J. Mental disorders in patients with fibromyalgia syndrome: screening in centres of different medical specialties. Schmerz 2013;27:296‐304. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc.), 2015.
Guyatt 2011 a
-
- Guyatt G, Oxman AD, Akl EA. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal Clinical Epidemiology 2011;64:383‐94. - PubMed
Guyatt 2011 b
-
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, et al. GRADE guidelines: 5. Rating the quality of evidence‐‐publication bias. Journal of Clinical Epidemiology 2011;64:1277‐82. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Häuser 2011
-
- Häuser W, Kosseva M, Üceyler N, Klose P, Sommer C. Emotional, physical, and sexual abuse in fibromyalgia syndrome: a systematic review with meta‐analysis. Arthritis Care & Research 2011;63:808‐20. - PubMed
Häuser 2013a
-
- Häuser W, Galek A, Erbslöh‐Möller B, Köllner V, Kühn‐Becker H, Langhorst J, et al. Posttraumatic stress disorder in fibromyalgia syndrome: prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain 2013;154:1216‐23. - PubMed
Häuser 2013b
Häuser 2014
-
- Häuser W, Henningsen P. Fibromyalgia syndrome ‐ a somaform disorder?. European Journal of Pain 2014;18:1052‐9. - PubMed
Häuser 2015a
Häuser 2015b
-
- Häuser W, Klose P, Welsch P, Petzke F, Nothacker M, et al. Methodology of the development of the updated LONTS guidelines for long‐term administration of opioids in noncancer pain. Schmerz 2015;29:8‐34. - PubMed
Kalso 2013
Kleinstäuber 2014
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. - PubMed
Lange 2010
-
- Lange M, Petermann F. Influence of depression on fibromyalgia: a systematic review. Schmerz 2010;24:326‐33. - PubMed
Langendam 2013
Lee 2012
-
- Lee YH, Choi SJ, Ji JD, Song GG. Candidate gene studies of fibromyalgia: a systematic review and meta‐analysis. Rheumatology International 2012;32:417‐26. - PubMed
Lunn 2014
McQuay 1998
-
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 978‐0192630483]
Mease 2009
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978–0–931092–69–5]
Moore 2009
Moore 2010a
-
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: ] - PubMed
Moore 2010b
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: ] - PMC - PubMed
Moore 2010c
Moore 2010d
-
- Moore RA, Smugar SS, Wang H, Peloso PM, Gammaitoni A. Numbers‐needed‐to‐treat analyses ‐ do timing, dropouts, and outcome matter? Pooled analysis of two randomized, placebo‐controlled chronic low back pain trials. Pain 2010;151(3):592‐7. [DOI: ] - PubMed
Moore 2011a
Moore 2011b
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: ] - PubMed
Moore 2011c
Moore 2012
Moore 2013a
Moore 2013b
Moore 2014
Moore 2015
Mork 2010
-
- Mork PJ, Vasseljen O, Nilsen TI. Association between physical exercise, body mass index, and risk of fibromyalgia: longitudinal data from the Norwegian Nord‐Trøndelag Health Study. Arthritis Care & Research 2010;62:611‐7. - PubMed
Mork 2012
-
- Mork PJ, Nilsen TI. Sleep problems and risk of fibromyalgia: longitudinal data on an adult female population in Norway. Arthritis and Rheumatism 2012;64:281‐4. - PubMed
Norman 2001
-
- Norman GR, Sridhar FG, Guyatt GH, Walter SD. Relation of distribution‐ and anchor‐based approaches in interpretation of changes in health‐related quality of life. Medical Care 2001;39:1039‐47. - PubMed
O'Brien 2010
Oaklander 2013
Okifuji 2015
Queiroz 2013
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rico‐Villademoros 2014
-
- Rico‐Villademoros F, Calandre EP, Slim M. Current status of atypical antipsychotics for the treatment of fibromyalgia. Drugs Today 2014;50:435‐44. - PubMed
Seidel 2013
Sommer 2012
-
- Sommer C, Häuser W, Burgmer M, Engelhardt R, Gerhold K, Petzke F, et al. Etiology and pathophysiology of fibromyalgia syndrome. Schmerz 2012;26:259‐67. - PubMed
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] - DOI - PMC - PubMed
Straube 2010
Straube 2011
Sultan 2008
Ventriglio 2015
Wang 2013
-
- Wang HR, Woo YS, Bahk WM. Atypical antipsychotics in the treatment of posttraumatic stress disorder. Clinical Neuropharmacology 2013;36:216‐22. - PubMed
Wiffen 2013
Wolfe 1990
-
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism 1990;33:160‐72. - PubMed
Wolfe 2010
-
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care & Research 2010;62:600‐10. - PubMed
Wolfe 2011
-
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. Journal of Rheumatology 2011;38:1113‐22. - PubMed
Wolfe 2013
Wolfe 2014
Yunus 2008
-
- Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Seminars in Arthritis and Rheumatism 2008;37:339‐52. - PubMed
Zhou 2015
Üçeyler 2013a
-
- Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel‐Schneider S, Schmid A, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013;136:e247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

