A Prospective Randomized Controlled Trial to Study the Impact of a Nutrition-Sensitive Intervention on Adult Women With Cancer Cachexia Undergoing Palliative Care in India
- PMID: 27252077
- PMCID: PMC5736069
- DOI: 10.1177/1534735416651968
A Prospective Randomized Controlled Trial to Study the Impact of a Nutrition-Sensitive Intervention on Adult Women With Cancer Cachexia Undergoing Palliative Care in India
Abstract
Purpose: Advanced cancer patients with disease progression develop cachexia. Nevertheless, cancer patients at nutritional risk have shown improved body weight and quality of life with oral nutritional supplements.
Method: This was a randomized controlled trial in adult female cancer patients (n = 63) attending palliative clinics, with symptoms of cachexia. Eligible patients were randomly distributed into control (n = 33) and intervention (n = 30) groups. Both groups were provided with nutritional and physical activity counseling, but the intervention group received an additional 100 g of Improved Atta (IAtta) for 6 months daily consumption. This study was designed to assess the efficacy of IAtta (with counseling) in enhancing the health status of cachexic patients. Anthropometric measurements, dietary intake, physical activity level and quality of life parameters were assessed at baseline, after 3 months, and at the end of 6 months.
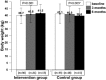
Results: Patients in the control group (n = 15) had significantly decreased body weight ( P = .003), mid-upper-arm circumference ( P = .002), and body fat ( P = .002) by the end of intervention. A trend of body weight gain in the intervention group (n = 17; P = .08) and significant increase of body fat ( P = .002) was observed; moreover, patients reported a significant improvement in fatigue ( P = .002) and appetite scores ( P = .006) under quality-of-life domains at the end of intervention.
Conclusions: Embedding a nutrition-sensitive intervention ( IAtta ) within Indian palliative care therapy may improve quality of life and stabilize body weight in cancer cachexia patients.
Trial registration: ClinicalTrials.gov NCT02350855.
Keywords: IAtta; cachexia; nutrition-sensitive intervention; palliative care; quality of life.
Conflict of interest statement
Figures



References
-
- Aapro M, Arends J, Bozzetti F, et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann Oncol. 2014;25:1492-1499. - PubMed
-
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489-495. - PubMed
-
- Radbruch L, Elsner F, Trottenberg P, Strasser F, Fearon K. Clinical Practice Guidelines on Cancer Cachexia in Advanced Cancer Patients With a Focus on Refractory Cachexia. Aachen, Germany: Department of Palliative Medicinen/European Palliative Care Research Collaborative; http://www.epcrc.org/getpublication2.php?id=ternkkdsszelxevzgtkb. Accessed March 10, 2016.
-
- Bauer JD, Ash S, Davidson WL, et al. Evidence based practice guidelines for the nutritional management of cancer cachexia. Nutr Diet. 2006;63(suppl 2):S3-S32.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

