A genomic approach to therapeutic target validation identifies a glucose-lowering GLP1R variant protective for coronary heart disease
- PMID: 27252175
- PMCID: PMC5219001
- DOI: 10.1126/scitranslmed.aad3744
A genomic approach to therapeutic target validation identifies a glucose-lowering GLP1R variant protective for coronary heart disease
Abstract
Regulatory authorities have indicated that new drugs to treat type 2 diabetes (T2D) should not be associated with an unacceptable increase in cardiovascular risk. Human genetics may be able to guide development of antidiabetic therapies by predicting cardiovascular and other health endpoints. We therefore investigated the association of variants in six genes that encode drug targets for obesity or T2D with a range of metabolic traits in up to 11,806 individuals by targeted exome sequencing and follow-up in 39,979 individuals by targeted genotyping, with additional in silico follow-up in consortia. We used these data to first compare associations of variants in genes encoding drug targets with the effects of pharmacological manipulation of those targets in clinical trials. We then tested the association of those variants with disease outcomes, including coronary heart disease, to predict cardiovascular safety of these agents. A low-frequency missense variant (Ala316Thr; rs10305492) in the gene encoding glucagon-like peptide-1 receptor (GLP1R), the target of GLP1R agonists, was associated with lower fasting glucose and T2D risk, consistent with GLP1R agonist therapies. The minor allele was also associated with protection against heart disease, thus providing evidence that GLP1R agonists are not likely to be associated with an unacceptable increase in cardiovascular risk. Our results provide an encouraging signal that these agents may be associated with benefit, a question currently being addressed in randomized controlled trials. Genetic variants associated with metabolic traits and multiple disease outcomes can be used to validate therapeutic targets at an early stage in the drug development process.
Copyright © 2016, American Association for the Advancement of Science.
Figures


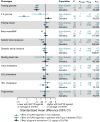

References
-
- US FDA. Guidance for Industry Diabetes Mellitus — Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. 2008 http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformati....
-
- Plenge RM, Scolnick EM, Altshuler D. Validating therapeutic targets through human genetics. Nat Rev Drug Discov. 2013;12:581–594. - PubMed
-
- Nelson MR, Tipney H, Painter JL, Shen J, Nicoletti P, Shen Y, Floratos A, Sham PC, Li MJ, Wang J, Cardon LR, Whittaker JC, Sanseau P. The support of human genetic evidence for approved drug indications. Nat Genet. 2015;47:856–860. - PubMed
-
- Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart AFR, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PIW, Klungel OH, Maitland-van der Zee AH, Peters BJM, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VHM, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WMM, a Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox Ka, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–80. - PMC - PubMed
-
- Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–1908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 17528/CRUK_/Cancer Research UK/United Kingdom
- HHSN268201100010C/HL/NHLBI NIH HHS/United States
- R01 DK072193/DK/NIDDK NIH HHS/United States
- MC_UU_12012/5/MRC_/Medical Research Council/United Kingdom
- R01 AG033193/AG/NIA NIH HHS/United States
- MR/L023784/1/MRC_/Medical Research Council/United Kingdom
- K24 DK080140/DK/NIDDK NIH HHS/United States
- 15007/CRUK_/Cancer Research UK/United Kingdom
- U01 DK078616/DK/NIDDK NIH HHS/United States
- MR/M008975/1/MRC_/Medical Research Council/United Kingdom
- 001/WHO_/World Health Organization/International
- 16465/CRUK_/Cancer Research UK/United Kingdom
- R01 DK093757/DK/NIDDK NIH HHS/United States
- R01 DK078616/DK/NIDDK NIH HHS/United States
- HHSN268201100012C/HL/NHLBI NIH HHS/United States
- MR/K013041/1/MRC_/Medical Research Council/United Kingdom
- G0800270/MRC_/Medical Research Council/United Kingdom
- 16491/CRUK_/Cancer Research UK/United Kingdom
- UL1 RR025005/RR/NCRR NIH HHS/United States
- HHSN268201100008C/HL/NHLBI NIH HHS/United States
- U01 AG032984/AG/NIA NIH HHS/United States
- R01 HL059367/HL/NHLBI NIH HHS/United States
- HHSN268201100007C/HL/NHLBI NIH HHS/United States
- RG/08/014/24067/BHF_/British Heart Foundation/United Kingdom
- HHSN268201100011C/HL/NHLBI NIH HHS/United States
- R01 HL086694/HL/NHLBI NIH HHS/United States
- U01 HG004402/HG/NHGRI NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- G0902227/MRC_/Medical Research Council/United Kingdom
- UL1 TR001108/TR/NCATS NIH HHS/United States
- MC_UU_12015/1/MRC_/Medical Research Council/United Kingdom
- MR/L501517/1/MRC_/Medical Research Council/United Kingdom
- P30 DK063491/DK/NIDDK NIH HHS/United States
- HHSN268201100006C/HL/NHLBI NIH HHS/United States
- R01 AG008122/AG/NIA NIH HHS/United States
- U01 DK062370/DK/NIDDK NIH HHS/United States
- 9675/CRUK_/Cancer Research UK/United Kingdom
- HHSN268201100009C/HL/NHLBI NIH HHS/United States
- HHSN268201100005C/HL/NHLBI NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- N01 RC037004/RC/CCR NIH HHS/United States
- 16942/CRUK_/Cancer Research UK/United Kingdom
- MR/L010305/1/MRC_/Medical Research Council/United Kingdom
- R01 HL087641/HL/NHLBI NIH HHS/United States
- N01 CN045165/CN/NCI NIH HHS/United States
- MR/L023784/2/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

