Solution Structures of Complement C2 and Its C4 Complexes Propose Pathway-specific Mechanisms for Control and Activation of the Complement Proconvertases
- PMID: 27252379
- PMCID: PMC4974366
- DOI: 10.1074/jbc.M116.722017
Solution Structures of Complement C2 and Its C4 Complexes Propose Pathway-specific Mechanisms for Control and Activation of the Complement Proconvertases
Abstract
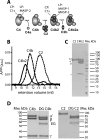
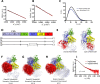
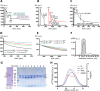
The lectin (LP) and classical (CP) pathways are two of the three main activation cascades of the complement system. These pathways start with recognition of different pathogen- or danger-associated molecular patterns and include identical steps of proteolytic activation of complement component C4, formation of the C3 proconvertase C4b2, followed by cleavage of complement component C2 within C4b2 resulting in the C3 convertase C4b2a. Here, we describe the solution structures of the two central complexes of the pathways, C3 proconvertase and C3 convertase, as well as the unbound zymogen C2 obtained by small angle x-ray scattering analysis. We analyzed both native and enzymatically deglycosylated C4b2 and C2 and showed that the resulting structural models were independent of the glycans. The small angle x-ray scattering-derived models suggest a different activation mode for the CP/LP C3 proconvertase as compared with that established for the alternative pathway proconvertase C3bB. This is likely due to the rather different structural and functional properties of the proteases activating the proconvertases. The solution structure of a stabilized form of the active CP/LP C3 convertase C4b2a is strikingly similar to the crystal structure of the alternative pathway C3 convertase C3bBb, which is in accordance with their identical functions in cleaving the complement proteins C3 and C5.
Keywords: complement system; convertase; innate immunity; protease; small angle x-ray scattering (SAXS); structural biology.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Kojouharova M., Reid K., and Gadjeva M. (2010) New insights into the molecular mechanisms of classical complement activation. Mol. Immunol. 47, 2154–2160 - PubMed
-
- Kjaer T. R., Thiel S., and Andersen G. R. (2013) Toward a structure-based comprehension of the lectin pathway of complement. Mol. Immunol. 56, 222–231 - PubMed
-
- Vorup-Jensen T., Petersen S. V., Hansen A. G., Poulsen K., Schwaeble W., Sim R. B., Reid K. B., Davis S. J., Thiel S., and Jensenius J. C. (2000) Distinct pathways of mannan-binding lectin (MBL)- and C1-complex autoactivation revealed by reconstitution of MBL with recombinant MBL-associated serine protease-2. J. Immunol. 165, 2093–2100 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

