Aminoglycosides: An Overview
- PMID: 27252397
- PMCID: PMC4888811
- DOI: 10.1101/cshperspect.a027029
Aminoglycosides: An Overview
Abstract
Aminoglycosides are natural or semisynthetic antibiotics derived from actinomycetes. They were among the first antibiotics to be introduced for routine clinical use and several examples have been approved for use in humans. They found widespread use as first-line agents in the early days of antimicrobial chemotherapy, but were eventually replaced in the 1980s with cephalosporins, carbapenems, and fluoroquinolones. Aminoglycosides synergize with a variety of other antibacterial classes, which, in combination with the continued increase in the rise of multidrug-resistant bacteria and the potential to improve the safety and efficacy of the class through optimized dosing regimens, has led to a renewed interest in these broad-spectrum and rapidly bactericidal antibacterials.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
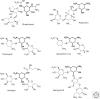
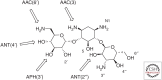

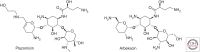
References
-
- Abis GS, Stockmann HB, van Egmond M, Bonjer HJ, Vandenbroucke-Grauls CM, Oosterling SJ. 2013. Selective decontamination of the digestive tract in gastrointestinal surgery: Useful in infection prevention? A systematic review. J Gastrointest Surg 17: 2172–2178. - PubMed
-
- Aínsa JA, Pérez E, Pelicic V, Berthet FX, Gicquel B, Martín C. 1997. Aminoglycoside 2′-N-acetyltransferase genes are universally present in mycobacteria: Characterization of the aac(2′)-Ic gene from Mycobacterium tuberculosis and the aac(2′)-Id gene from Mycobacterium smegmatis. Mol Microbiol 24: 431–441. - PubMed
-
- Almaghrabi R, Clancy CJ, Doi Y, Hao B, Chen L, Shields RK, Press EG, Iovine NM, Townsend BM, Wagener MM, et al. 2014. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother 58: 4443–4451. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
