Multi-Target Directed Donepezil-Like Ligands for Alzheimer's Disease
- PMID: 27252617
- PMCID: PMC4879129
- DOI: 10.3389/fnins.2016.00205
Multi-Target Directed Donepezil-Like Ligands for Alzheimer's Disease
Abstract
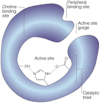
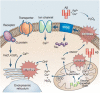
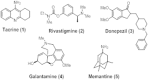
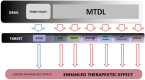
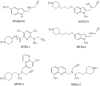
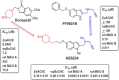
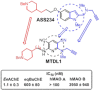
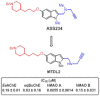
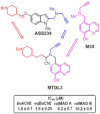
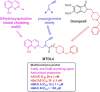
HIGHLIGHTS ASS234 is a MTDL compound containing a moiety from Donepezil and the propargyl group from the PF 9601N, a potent and selective MAO B inhibitor. This compound is the most advanced anti-Alzheimer agent for preclinical studies identified in our laboratory.Derived from ASS234 both multipotent donepezil-indolyl (MTDL-1) and donepezil-pyridyl hybrids (MTDL-2) were designed and evaluated as inhibitors of AChE/BuChE and both MAO isoforms. MTDL-2 showed more high affinity toward the four enzymes than MTDL-1.MTDL-3 and MTDL-4, were designed containing the N-benzylpiperidinium moiety from Donepezil, a metal- chelating 8-hydroxyquinoline group and linked to a N-propargyl core and they were pharmacologically evaluated.The presence of the cyano group in MTDL-3, enhanced binding to AChE, BuChE and MAO A. It showed antioxidant behavior and it was able to strongly complex Cu(II), Zn(II) and Fe(III).MTDL-4 showed higher affinity toward AChE, BuChE.MTDL-3 exhibited good brain penetration capacity (ADMET) and less toxicity than Donepezil. Memory deficits in scopolamine-lesioned animals were restored by MTDL-3.MTDL-3 particularly emerged as a ligand showing remarkable potential benefits for its use in AD therapy. Alzheimer's disease (AD), the most common form of adult onset dementia, is an age-related neurodegenerative disorder characterized by progressive memory loss, decline in language skills, and other cognitive impairments. Although its etiology is not completely known, several factors including deficits of acetylcholine, β-amyloid deposits, τ-protein phosphorylation, oxidative stress, and neuroinflammation are considered to play significant roles in the pathophysiology of this disease. For a long time, AD patients have been treated with acetylcholinesterase inhibitors such as donepezil (Aricept®) but with limited therapeutic success. This might be due to the complex multifactorial nature of AD, a fact that has prompted the design of new Multi-Target-Directed Ligands (MTDL) based on the "one molecule, multiple targets" paradigm. Thus, in this context, different series of novel multifunctional molecules with antioxidant, anti-amyloid, anti-inflammatory, and metal-chelating properties able to interact with multiple enzymes of therapeutic interest in AD pathology including acetylcholinesterase, butyrylcholinesterase, and monoamine oxidases A and B have been designed and assessed biologically. This review describes the multiple targets, the design rationale and an in-house MTDL library, bearing the N-benzylpiperidine motif present in donepezil, linked to different heterocyclic ring systems (indole, pyridine, or 8-hydroxyquinoline) with special emphasis on compound ASS234, an N-propargylindole derivative. The description of the in vitro biological properties of the compounds and discussion of the corresponding structure-activity-relationships allows us to highlight new issues for the identification of more efficient MTDL for use in AD therapy.
Keywords: Alzheimer's disease; anti-β-amyloid aggregation; donepezil; multi-target-directed ligands; oxidative stress.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

