Single Neurons in the Insular Cortex of a Macaque Monkey Respond to Skin Brushing: Preliminary Data of the Possible Representation of Pleasant Touch
- PMID: 27252631
- PMCID: PMC4877530
- DOI: 10.3389/fnbeh.2016.00090
Single Neurons in the Insular Cortex of a Macaque Monkey Respond to Skin Brushing: Preliminary Data of the Possible Representation of Pleasant Touch
Abstract
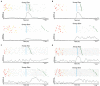
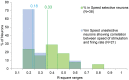
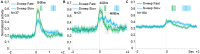
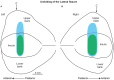
Pleasant touch may serve as a foundation for affiliative behavior, providing a mechanism for the formation and maintenance of social bonds among conspecifics. In humans, this touch is usually referred to as the caress. Dynamic caressing performed on the hairy skin with a velocity of 1-10 cm/s is perceived as being pleasant and determines positive cardio-physiological effects. Furthermore, imaging human studies show that affiliative touch activates the posterior insular cortex (pIC). Recently, it was demonstrated that pleasant touch in monkeys (i.e., sweeping in a grooming-like manner) is performed with velocities similar to those characteristics of human caress (9.31 cm/s), and causes similarly positive autonomic effects, if performed with velocity of 5 cm/s and 10 cm/s, but not lower or higher. Due to similarities between the human caress and non-human primate sweeping, we investigated for the first time whether single neurons of the perisylvian regions (secondary somatosensory cortex [SII] and pIC) of a rhesus monkey can process sweeping touch differently depending on the stimulus speed. We applied stimulation with two speeds: one that optimally induces positive cardio-physiological effects in the monkey who receives it, and includes the real speed of sweep (5-15 cm/s, sweep fast), and a non-optimal speed (1-5 cm/s, sweep slow). The results show that single neurons of insular cortex differently encode the stimulus speed. In particular, even the majority of recorded somatosensory neurons (82.96%) did not discriminate the two speeds, a small set of neurons (16.59%) were modulated just during the sweep fast. These findings represent the first evidence that single neurons of the non-human primates insular cortex can code affiliative touch, highlighting the similarity between human and non-human primates' social touch systems. This study constitutes an important starting point to carry out deeper investigation on neuronal processing of pleasant sweeping in the central nervous system.
Keywords: Macaca mulatta; grooming; insular cortex; perisylvian region; pleasant touch; single neurons.
Figures



References
-
- Bessou P., Burgess P. R., Perl E. R., Taylor C. B. (1971). Dynamic properties of mechanoreceptors with unmyelinated (C) fibers. J. Neurophysiol. 34, 116–131. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

