Degradation characteristics, cell viability and host tissue responses of PDLLA-based scaffold with PRGD and β-TCP nanoparticles incorporation
- PMID: 27252885
- PMCID: PMC4881616
- DOI: 10.1093/rb/rbw017
Degradation characteristics, cell viability and host tissue responses of PDLLA-based scaffold with PRGD and β-TCP nanoparticles incorporation
Abstract
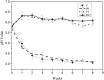
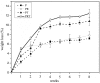
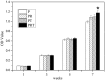
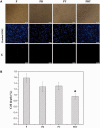
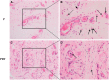
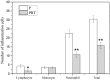
This study is aimed to evaluate the degradation characteristics, cell viability and host tissue responses of PDLLA/PRGD/β-TCP (PRT) composite nerve scaffold, which was fabricated by poly(d, l-lactic acid) (PDLLA), RGD peptide(Gly-Arg-Gly-Asp-Tyr, GRGDY, abbreviated as RGD) modified poly-{(lactic acid)-co-[(glycolic acid)-alt-(l-lysine)]}(PRGD) and β-tricalcium phosphate (β-TCP). The scaffolds' in vitro degradation behaviors were investigated in detail by analysing changes in weight loss, pH and morphology. Then, the 3-(4,5-dimethyl-2-thiazolyl) -2,5-diphenyl-2 -H-tetrazolium bromide (MTT) assay and cell live/dead assay were carried out to assess their cell viability. Moreover, in vivo degradation patterns and host inflammation responses were monitored by subcutaneous implantation of PRT scaffold in rats. Our data showed that, among the tested scaffolds, the PRT scaffold had the best buffering capacity (pH = 6.1-6.3) and fastest degradation rate (12.4%, 8 weeks) during in vitro study, which was contributed by the incorporation of β-TCP nanoparticles. After in vitro and in vivo degradation, the high porosity structure of PRT could be observed using scanning electron microscopy. Meanwhile, the PRT scaffold could significantly promote cell survival. In the PRT scaffold implantation region, less inflammatory cells (especially for neutrophil and lymphocyte) could be detected. These results indicated that the PRT composite scaffold had a good biodegradable property; it could improve cells survival and reduced the adverse host tissue inflammation responses.
Keywords: PDLLA/PRGD/β-TCP scaffold; cell viability; degradation; host tissue responses.
Figures


References
-
- Pfister BJ, Gordon T, Loverde JR. et al. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng 2011;39:81–124. - PubMed
-
- Stang F, Keilhoff G, Fansa H. Biocompatibility of different nerve tubes. Materials 2009;2:1480–507.
-
- Evans GRD. Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat Rec 2001;263:396–404. - PubMed
-
- Meek MF, Coert JH. Clinical use of nerve conduits in peripheral-nerve repair: review of the literature. J Reconstr Microsurg 2002;18:97–109. - PubMed
-
- Luo LH, Gan L, Liu YM. et al. Construction of nerve guide conduits from cellulose/soy protein composite membranes combined with Schwann cells and pyrroloquinoline quinone for the repair of peripheral nerve defect. Biochem Biophys Res Commun 2015;457:507–13. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

