A mitofusin-dependent docking ring complex triggers mitochondrial fusion in vitro
- PMID: 27253069
- PMCID: PMC4929004
- DOI: 10.7554/eLife.14618
A mitofusin-dependent docking ring complex triggers mitochondrial fusion in vitro
Abstract
Fusion of mitochondrial outer membranes is crucial for proper organelle function and involves large GTPases called mitofusins. The discrete steps that allow mitochondria to attach to one another and merge their outer membranes are unknown. By combining an in vitro mitochondrial fusion assay with electron cryo-tomography (cryo-ET), we visualize the junction between attached mitochondria isolated from Saccharomyces cerevisiae and observe complexes that mediate this attachment. We find that cycles of GTP hydrolysis induce progressive formation of a docking ring structure around extended areas of contact. Further GTP hydrolysis triggers local outer membrane fusion at the periphery of the contact region. These findings unravel key features of mitofusin-dependent fusion of outer membranes and constitute an important advance in our understanding of how mitochondria connect and merge.
Keywords: S. cerevisiae; biophysics; cell biology; cryo-EM; dynamin-related-proteins; membrane fusion; mitochondria; mitochondrial dynamics; mitofusin; structural biology.
Conflict of interest statement
WK: Reviewing editor,
The other authors declare that no competing interests exist.
Figures
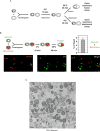



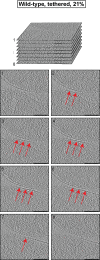





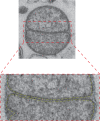

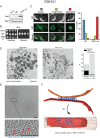





References
-
- Abutbul-Ionita I, Rujiviphat J, Nir I, McQuibban GA, Danino D. Membrane tethering and nucleotide-dependent conformational changes drive mitochondrial genome maintenance (Mgm1) protein-mediated membrane fusion. The Journal of Biological Chemistry. 2012;287:36634–36638. doi: 10.1074/jbc.C112.406769. - DOI - PMC - PubMed
-
- Bian X, Klemm RW, Liu TY, Zhang M, Sun S, Sui X, Liu X, Rapoport TA, Hu J. Structures of the atlastin GTPase provide insight into homotypic fusion of endoplasmic reticulum membranes. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3976–3981. doi: 10.1073/pnas.1101643108. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

