Small RNA-Based Antiviral Defense in the Phytopathogenic Fungus Colletotrichum higginsianum
- PMID: 27253323
- PMCID: PMC4890784
- DOI: 10.1371/journal.ppat.1005640
Small RNA-Based Antiviral Defense in the Phytopathogenic Fungus Colletotrichum higginsianum
Abstract
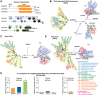
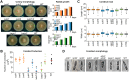
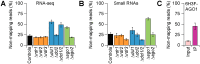
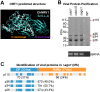
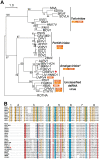
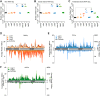
Even though the fungal kingdom contains more than 3 million species, little is known about the biological roles of RNA silencing in fungi. The Colletotrichum genus comprises fungal species that are pathogenic for a wide range of crop species worldwide. To investigate the role of RNA silencing in the ascomycete fungus Colletotrichum higginsianum, knock-out mutants affecting genes for three RNA-dependent RNA polymerase (RDR), two Dicer-like (DCL), and two Argonaute (AGO) proteins were generated by targeted gene replacement. No effects were observed on vegetative growth for any mutant strain when grown on complex or minimal media. However, Δdcl1, Δdcl1Δdcl2 double mutant, and Δago1 strains showed severe defects in conidiation and conidia morphology. Total RNA transcripts and small RNA populations were analyzed in parental and mutant strains. The greatest effects on both RNA populations was observed in the Δdcl1, Δdcl1Δdcl2, and Δago1 strains, in which a previously uncharacterized dsRNA mycovirus [termed Colletotrichum higginsianum non-segmented dsRNA virus 1 (ChNRV1)] was derepressed. Phylogenetic analyses clearly showed a close relationship between ChNRV1 and members of the segmented Partitiviridae family, despite the non-segmented nature of the genome. Immunoprecipitation of small RNAs associated with AGO1 showed abundant loading of 5'U-containing viral siRNA. C. higginsianum parental and Δdcl1 mutant strains cured of ChNRV1 revealed that the conidiation and spore morphology defects were primarily caused by ChNRV1. Based on these results, RNA silencing involving ChDCL1 and ChAGO1 in C. higginsianum is proposed to function as an antiviral mechanism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

