Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering
- PMID: 27254107
- PMCID: PMC5201005
- DOI: 10.1002/smll.201600178
Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering
Abstract
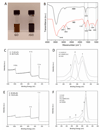
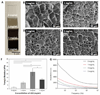
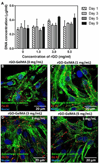
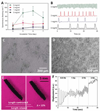
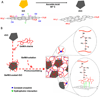
Biomaterials currently used in cardiac tissue engineering have certain limitations, such as lack of electrical conductivity and appropriate mechanical properties, which are two parameters playing a key role in regulating cardiac cell behavior. Here, the myocardial tissue constructs are engineered based on reduced graphene oxide (rGO)-incorporated gelatin methacryloyl (GelMA) hybrid hydrogels. The incorporation of rGO into the GelMA matrix significantly enhances the electrical conductivity and mechanical properties of the material. Moreover, cells cultured on composite rGO-GelMA scaffolds exhibit better biological activities such as cell viability, proliferation, and maturation compared to ones cultured on GelMA hydrogels. Cardiomyocytes show stronger contractility and faster spontaneous beating rate on rGO-GelMA hydrogel sheets compared to those on pristine GelMA hydrogels, as well as GO-GelMA hydrogel sheets with similar mechanical property and particle concentration. Our strategy of integrating rGO within a biocompatible hydrogel is expected to be broadly applicable for future biomaterial designs to improve tissue engineering outcomes. The engineered cardiac tissue constructs using rGO incorporated hybrid hydrogels can potentially provide high-fidelity tissue models for drug studies and the investigations of cardiac tissue development and/or disease processes in vitro.
Keywords: bioactuator; cardiac tissue engineering; gelatin; hydrogel; reduced graphene oxide.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- Camelliti P, Borg TK, Kohl P. Cardiovasc Res. 2005;65:40. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

